0310
Noninvasive assessment of water content and collagen extent of liver tissue with multiparametric magnetic resonance elastography (MRE)1Radiology, Mayo Clinic, Rochester, MN, United States, 2Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, United States, 3the Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China, 4Research and Development, Takeda Pharmaceuticals International Co., Cambridge, MA, United States
Synopsis
Liver biopsy remains the gold standard for staging liver fibrosis. However, its invasive nature makes it unacceptable for long-term disease dynamic monitoring. In addition, current histopathological scoring systems for staging liver fibrosis are not quantitative. Also, the inflammatory response of increased interstitial fluid volume is precursory and occult with histological analysis. It is critical to address the overlooked fluid-associated inflammatory response and semi-quantitative fibrosis grading. Here, we use a novel technique, magnetic resonance elastography, combined with ALT, as a noninvasive quantitative method to quantify and monitor hepatic water and fibrosis content in a mouse model with varying disease progression/regression.
Introduction
Liver biopsy remains the gold standard for staging liver fibrosis1. However, its invasive nature makes it unacceptable for long-term dynamic monitoring of inflammation and fibrosis extent. In addition, the current histopathological scoring systems are semi-quantitative. Thus, the extent of fibrosis may differ even in the same fibrosis stage, especially in patients with F>=4, which may lead to underestimation of anti-fibrotic effect. Indeed, evidence has shown that quantitative assessment of liver fibrosis did not linearly associate with fibrosis stage2. Interstitial fluid volume augmentation (or edema) is a histologically occult process before inflammatory cellular invasion, which may result in changes of fluid-associated mechanical response, such as viscosity or loss modulus3,4. Magnetic resonance elastography (MRE) is a novel technique that can measure the mechanical properties of the liver. Studies have shown that liver stiffness measured by MRE correlates well with the progression of liver fibrosis stage5-8. However, whether MRE alone or in combination with liver function tests can disentangle and quantify liver inflammation and fibrosis remain unknown. Therefore, the purpose of this preclinical study was to evaluate the value of multiparametric MRE in combination with liver function tests in the assessment of liver inflammation and fibrosis progression and regression in a mouse model.Methods
A total of 35 wild-type C57BL/6J male mice were studied. Progression of liver fibrosis was induced by intraperitoneal injection of carbon tetrachloride (CCL4) twice a week for 2, 3, or 4 weeks. Regression of liver fibrosis was achieved by withdrawal of CCL4 for 2 or 4 weeks after a 4-week CCL4 treatment. Three corresponding age-matched control groups were included. 3D MRE at 80 and 200Hz were performed at different time points (baseline, week 2, 3, 4, 6, and 8) in each study group. Multiple mechanical parameters of the liver were calculated. Mice were weighed and sacrificed after the last MRE scanning. Serum samples were collected for liver function tests. Liver samples were collected for biochemistry/physical evaluation of liver fibrosis (hydroxyproline content) and inflammation (water content). The existence of liver fibrosis was evaluated histologically with Sirius red staining. The existence of hepatic inflammation was determined simply by untreated (i.e. age-matched controls) and treated (i.e., past administration of CCl4 including both progression and regression) groups. Spearman’s correlation, univariate and multivariable analyses were performed to identify the independent MRE and liver function predictors for hydroxyproline and water extent. Prediction models were generated by adaptive Lasso regression with AICc validation. Diagnostic accuracies were evaluated by ROC analysis for screening liver fibrosis and inflammation.Results
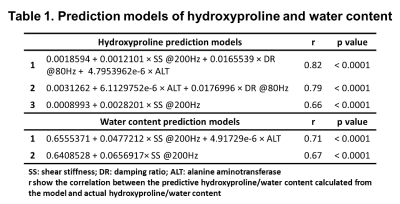
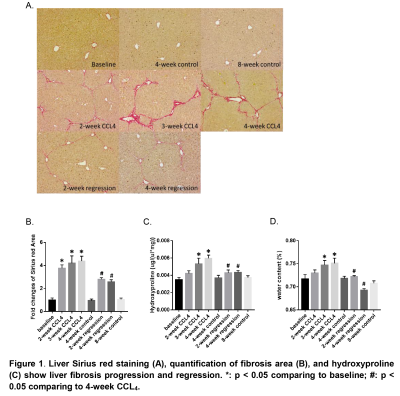
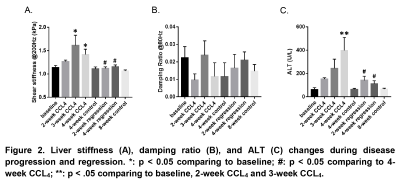
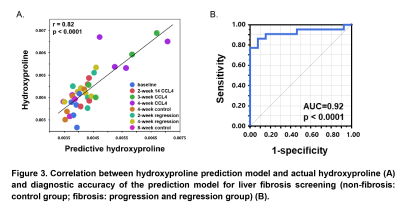
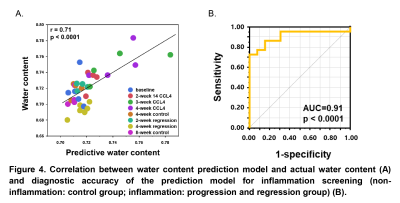
Mice liver hydroxyproline and water content increased progressively with time after the administration of CCl4 and decreased after CCl4 withdrawal (Figure 1). Liver shear stiffness (SS) and alanine aminotransferase (ALT) increased during disease progression and decreased during disease regression. However, damping ratio (DR) fluctuated in individual animals, and there was no significant difference between groups (Figure 2). In multivariate regression, SS, DR, and ALT were identified as significant predictors for hydroxyproline, while SS and ALT were identified as significant predictors for water content. Prediction models for liver hydroxyproline and water content with different combinations of predictors were shown in Table 1. A model combining SS, DR, and ALT to predict hydroxyproline showed the best correlation with actual hydroxyproline (r = 0.82, p < 0.0001). The diagnostic accuracy of predicted hydroxyproline for discriminating fibrosis from non-fibrosis was excellent (AUC = 0.92, p < 0.0001) (Figure 3). Comparing with SS alone, a model combining SS and ALT to predict liver water content showed better correlation with actual water content (r = 0.71, p < 0.0001). The diagnostic accuracy of predicted water content for differentiating diseased from controls was excellent (AUC = 0.91, p < 0.0001) (Figure 4).Discussion
It has been well established that liver stiffness can accurately detect the progression of liver fibrosis5-8. Our study found that liver stiffness decreased with the reduction of hepatic fibrosis, which reinforced the fact that liver stiffness is sensitive to the fibrosis content. Our study revealed that a model combining SS, DR, and ALT, instead of SS alone, can better quantify fibrosis content with concurrent inflammation. In our previous study, we found that DR was associated with liver inflammation9. The statistically insignificant group differences observed in DR in this study may be attributed to the biweekly administration of CCl4 and invasive liver needle penetration needed for MRE. This time-dependent CCL4-induced mouse model could have substantial fluctuation of inflammatory response in each individual animal over time. Therefore, the effects of DR and ALT, associated with liver inflammation or cellular injury, can be disentangled in the prediction of fibrosis extent. Inflammation or injury to the liver can lead to increase in the interstitial fluid volume and pressure, and the latter can increase liver stiffness3,4. Therefore, SS in combination with ALT can predict liver water content, a surrogate of inflammation.Conclusion
A multiparametric model combining SS and DR, derived from MRE, and ALT can serve as a noninvasive surrogate in detecting and monitoring the progression and regression of liver fibrosis and concurrent inflammation, which has the potential to noninvasively evaluate the efficacy of novel anti-fibrotic/inflammatory treatments in patients.Acknowledgements
This study is supported by NIBIB: EB017197 (M.Y.), NIBIB: EB001981 (R.L.E.), NIAAA: AA021171 (V.H.S.), and Takeda Pharmaceuticals International: FP00102673 (M.Y.).References
1. Li S, Sun X, Chen M et al. Liver Fibrosis Conventional and Molecular Imaging Diagnosis Update. J Liver 2019; 8
2. Masugi Y, Abe T, Tsujikawa H et al. Quantitative assessment of liver fibrosis reveals a nonlinear association with fibrosis stage in nonalcoholic fatty liver disease. Hepatol Commun 2018; 2: 58-68
3. Reed RK, Rubin K. Transcapillary exchange: role and importance of the interstitial fluid pressure and the extracellular matrix. Cardiovasc Res 2010; 87: 211-217
4. Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev 2012; 92: 1005-1060
5. Xiao G, Zhu S, Xiao X et al. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology 2017; 66: 1486-1501
6. Shi Y, Xia F, Li QJ et al. Magnetic Resonance Elastography for the Evaluation of Liver Fibrosis in Chronic Hepatitis B and C by Using Both Gradient-Recalled Echo and Spin-Echo Echo Planar Imaging: A Prospective Study. Am J Gastroenterol 2016; 111: 823-833
7. Park CC, Nguyen P, Hernandez C et al. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology 2017; 152: 598-607 e592
8. Fu F, Li X, Chen C et al. Non-invasive assessment of hepatic fibrosis: comparison of MR elastography to transient elastography and intravoxel incoherent motion diffusion-weighted MRI. Abdom Radiol (NY) 2019, DOI: 10.1007/s00261-019-02140-x
9. Yin M, Glaser KJ, Manduca A et al. Distinguishing between Hepatic Inflammation and Fibrosis with MR Elastography. Radiology 2017; 284: 694-705
Figures