0297
Multiple-echo steady-state for MR-only prostate radiotherapy: Combined T1/T2-weighted imaging, water-fat separation, and synthetic CT1Image Sciences Institute, UMC Utrecht, Utrecht, Netherlands, 2MRIguidance BV, Utrecht, Netherlands
Synopsis
We propose an efficient sequence for the acquisition of multiple contrasts for MR-only radiotherapy. By including T2-weighted echoes to a gradient echo sequence, this sequence provides T1- and T2-weighted imaging and water-fat separation in a single 4 minute acquisition. A previously trained deep neural network for synthetic CT generation was successfully applied to this new sequence, demonstrating that synthetic CT-based dose calculations can be performed. Furthermore, increased contrast between the anatomies of interest shows promise for (automatic) segmentation.
Introduction
In radiotherapy, MRI is often combined with CT imaging to provide both accurate delineation of organs at risk and accurate dose calculation. However, such a multimodal workflow is costly, has high patient burden, and is labor intensive, which would be alleviated with an MR-only workflow. Such a workflow requires a comprehensive scan protocol with various contrasts, such as T1-, T2-, and diffusion-weighted images.Deep learning-based synthetic CT generation from MR images have been shown to be accurate enough to replace CT images for dose calculations1. T1-weighted multi-echo gradient echo (GRE) scans with Dixon water-fat separation have been shown to be well-suited for this purpose1,2. In addition, GRE scans contain valuable information about susceptibility, which has been shown to be useful for localization of gold fiducials that are used for radiotherapy planning3. However, for delineation of organs and cancer staging, T2-weighted images and T2 mapping are of interest4. The double-echo state-state (DESS) sequence was recently shown to be a time-efficient alternative to spin-echo-based T2-weighted imaging and T2 mapping in the prostate4. In this study we demonstrate a combination of the GRE sequence with DESS to include T2-weighted echoes for better organ delineation, while maintaining the GRE echoes for tasks such as synthetic CT generation.
Methods
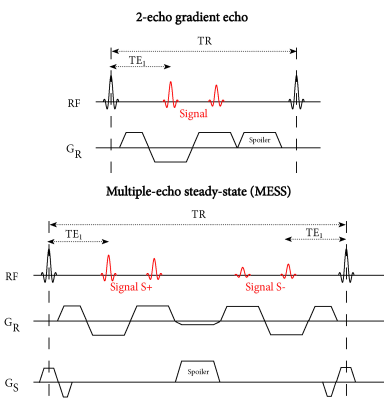
Acquisition:Our multiple-echo steady-state (MESS) sequence extends a 2-echo GRE scan with two additional echoes with a DESS acquisition scheme (Figure 1). Both sets of two echoes are suitable for Dixon water-fat separation, where the added echoes have increased T2-weighting.
We implemented the MESS sequence on a 3T scanner (Philips Ingenia, Best, The Netherlands), and acquired 2-echo GRE and MESS images of the pelvis for one healthy volunteer (male, age 40). The parameters for the GRE sequence were: TE1/TE2/TR 2.1/3.5/6.5, flip angle 10, resolution $$$1.2\times1.2\times2$$$ mm, FOV $$$436\times280\times160$$$ mm, $$$1.2\times1.1$$$ SENSE acceleration, bandwidth 1122 Hz/pixel, scan duration 157 seconds. The parameters for the 4-echo MESS sequence were matched to the GRE sequence, with the exception of: TE1/TE2/TE3/TE4/TR 2.1/3.5/6.3/7.7/9.8, bandwidth 869 Hz/pixel, scan duration 238 seconds.
Water-fat separation:
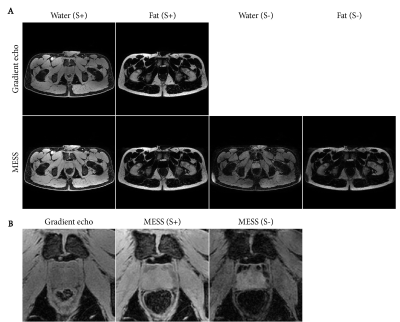
We performed two 2-point Dixon water-fat separations on the MESS acquisition, for the first two echoes and for the last two echoes. An analytical 2-point water-fat separation5 was used, where the field phasor was determined using region growing and local smoothing on the first pair of echoes. The field phasor was re-used to initialize the separation on the second pair of echoes, which should prevent inconsistent water-fat flips in both water-fat separations.
Synthetic CT:
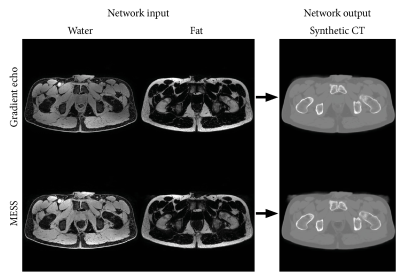
For synthetic CT generation of the GRE and MESS scans we used a 3D patch-based convolutional neural network that was previously trained on 25 prostate cancer patients, where the water and fat reconstructions of an identical GRE sequence were used as input channels2. This network was applied without modification to the water and fat images of both the GRE and MESS scans.
Results
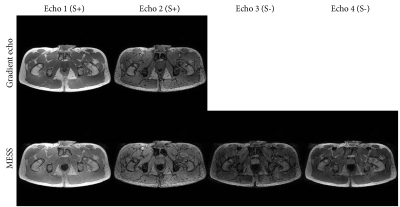
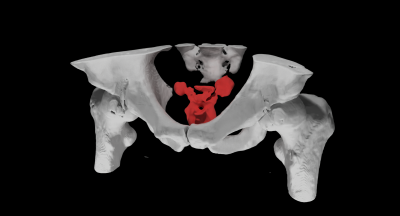
Figure 2 shows the acquired MESS images in comparison with the GRE images. Figure 3 shows the water-fat separated images. The first two echoes of both sequences have a close resemblance, with a minor difference in T1-weighting due to the modified repetition time. The 3rd and 4th MESS echoes show increased T2 contrast. This increased contrast is especially apparent in the prostate (Figure 3B), which stands out against the tissues surrounding it, providing valuable information for its delineation.The synthetic CT images generated from the GRE and MESS images are shown in Figure 4, demonstrating that the synthetic CT generation network can be applied successfully to MESS images, despite not being trained on them. The synthetic CT image from MESS is visualized in 3D in Figure 5, with a simple threshold-based segmentation of the prostate and seminal vesicles from the T2-weighted MESS water image.
Discussion & Conclusion
By combining a multi-echo gradient echo and DESS sequence, MESS efficiently provides both T1- and T2-weighted contrasts and water-fat separation. Because these contrasts are acquired by the same sequence, the images are intrinsically registered and in the same resolution, which simplifies MR-only workflows by removing the need for inter-scan registration. By matching the MESS acquisition parameters to a GRE sequence that is typically included in clinical examinations, deep learning-based synthetic CT generation was applicable directly to MESS images, without need for training a new model.The inclusion of T2-weighted contrast provides more information to distinguish organs (e.g. the prostate and seminal vesicles, Figure 5) which benefits segmentation of organs for radiotherapy planning. Although in theory the T2 contrast from DESS can be used for T2 mapping in the prostate4, in this study we chose a relatively short repetition time to mimic the GRE sequence, which limited the T2 contrast. If that requirement is dropped, for example if enough MESS training data is available for synthetic CT training, then the scan parameters of the MESS sequence could be changed to optimize SNR and T2 contrast by extending the repetition time and increasing the flip angle.
In 4 minutes, the MESS sequence acquired multiple contrasts, which shows promise for MR-only radiotherapy workflows that require organ segmentation and synthetic CT for dose calculations.
Acknowledgements
This work is part of the research programme Applied and Engineering Sciences (TTW) with project number 15479 which is (partly) financed by the Netherlands Organization for Scientific Research (NWO).References
1. Maspero M, Savenije MHF, Dinkla AM, et al. Dose evaluation of fast synthetic-CT generation using a generative adversarial network for general pelvis MR-only radiotherapy. Phys. Med. Biol. 2018;63(18):185001.
2.
Florkow MC, Zijlstra F, Willemsen K, et al. Deep learning–based MR-to-CT
synthesis: The influence of varying gradient echo–based MR images as input
channels. Magn. Reson. Med. 2019. Early view online.
3. Maspero M, Berg CAT van den, Zijlstra F, et al. Evaluation of an automatic MR-based gold fiducial marker localisation method for MR-only prostate radiotherapy. Phys. Med. Biol. 2017;62(20):7981–8002.
4. Dregely I, Margolis DAJ, Sung K, et al. Rapid quantitative T2 mapping of the prostate using three-dimensional dual echo steady state MRI at 3T. Magn. Reson. Med. 2016;76(6):1720–1729.
5. Berglund J, Ahlström H, Johansson L, et al. Two-point dixon method with flexible echo times. Magn. Reson. Med. 2011;65(4):994–1004.
Figures