0294
A comprehensive distortion-free 2-minute brain MR examination using BUDA and Wave-CAIPI1Siemens Medical Solutions, Boston, MA, United States, 2Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Boston, MA, United States, 3Department of Radiology, Massachusetts General Hospital, Boston, MA, United States, 4Harvard Medical School, Boston, MA, United States, 5Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Boston, MA, United States, 6Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China, 7Center for Brain Imaging Science and Technology, Biomedical Engineering, Zhejiang University, Hangzhou, Zhejiang, China, 8State Key Laboratory of Modern Optical Instrumentation, College of Optical Science and Engineering, Zhejiang University, Hangzhou, Zhejiang, China, 9Siemens Healtcare GmbH, Erlangen, Germany, 10Department of Physics and Astronomy, Heidelberg University, Heidelberg, Germany
Synopsis
We utilize Wave-CAIPI and BUDA techniques to develop a rapid 2-minute protocol that produces high in-plane resolution and distortion-free axial images for comprehensive evaluation of the brain. The protocol includes 3D T1-weighted Wave-CAIPI MPRAGE, 3D dark-fluid T2-weighted Wave-CAIPI SPACE-FLAIR, 2D T2*-weighted gradient echo BUDA, 2D T2-weighted and diffusion-weighted spin-echo BUDA. The results of the optimized protocol demonstrate comparable image quality, tissue contrast, and spatial resolution to standard clinical scans while keeping the total scan time to less than 2 minutes. The advanced acquisition and reconstruction framework presented here offers a path toward increasing clinical acceptance of ultrafast brain examinations.
INTRODUCTION
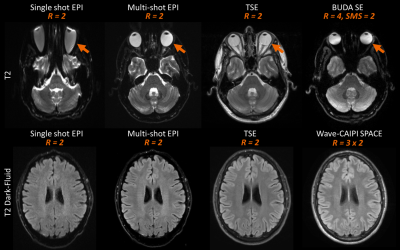
The long acquisition times for routine brain MRI examinations can present a significant barrier to evaluating particular clinical populations such as pediatric and uncooperative patients. Echo planar imaging (EPI) has been widely used in many clinical protocols to reduce imaging time and decrease motion artifacts. However, EPI is particularly prone to geometric distortions that may limit diagnostic accuracy. Alternatively, the fast MRI techniques Wave-CAIPIRINHA (Wave-CAIPI) [1, 2] for 3D examinations and Blip Up-Down Acquisition (BUDA) [3] for EPI examinations, provide robust acquisition and reconstruction frameworks that achieve distortion-free, high-resolution MRI for different applications while shortening scan time. Each of these imaging approaches offers unique advantages and drawbacks, as illustrated in Figure 1. Building on these strategies, we develop a highly efficient comprehensive 2-minute protocol that combines the advantages of the Wave-CAIPI and BUDA frameworks to achieve axial thick-slice 2D-like MRI images with high in-plane resolution and without distortion to match the image quality typically represented in the majority of routine brain MRI examinations. This protocol has the potential to provide comparable image quality and image contrast to the standard exam for the evaluation of intracranial pathology.METHODS
This study was approved by the IRB and was HIPAA compliant. As a proof of principle, MRI data was acquired on a single healthy volunteer on a 3T MRI scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with a 32-channel multi-array receiver coils.The imaging protocols included:
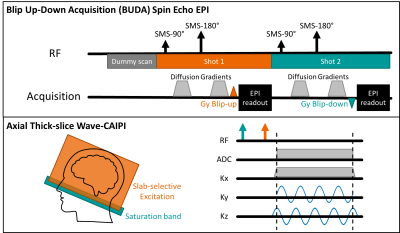
(i) Wave-protocol: A prototype 3D Wave-CAIPI MPRAGE for T1-weighted images (TR/TE: 2500/4.35ms; TI: 1110ms; acceleration factor (R): 3x3, FOV: 256x232x144mm3; resolution: 1x1x4 mm3) and a prototype 3D Wave-CAIPI SPACE-FLAIR for dark-fluid T2-weighted images (TR/TE: 5000/391ms; TI: 1800ms; R: 3x2, FOV: 224x224x144mm3; resolution: 0.88x0.88x3 mm3) were acquired. Slab-selective excitation and saturation band were used to acquire data in the axial plane. To avoid signal wrapping, 90% of slab thickness was excited for Wave-CAIPI SPACE-FLAIR (Figure 2). Wave-CAIPI SPACE-FLAIR was reformatted to 0.88x0.88x4mm3 for subsequent comparisons. An efficient sampling order for Wave-CAIPI MPRAGE was achieved by progressing along the phase encoding first followed by the partition encodings.
(ii) BUDA-protocol: A prototype 2D gradient echo (GRE) BUDA for T2*-weighted images (TR/TE: 2900/29ms; R: 4, FOV: 256x256x144mm3; resolution: 1x1x4 mm3) and a prototype 2D spin-echo (SE) BUDA for T2-weighted and diffusion-weighted images (DWI) (TR/TE: 3000/60ms; R: 4, multiband factor (SMS): 2, FOV: 256x256x144mm3; resolution: 1x1x4 mm3, phase partial Fourier (PF): 6/8, b-value: 0, 1000 s/mm2 with 6 directions) were acquired. In addition, a fast distortion-free GRE prescan was also acquired to perform coil sensitivities estimation for BUDA. Figure 2 shows the sequence diagram of SE EPI with BUDA, where the interleaved blip-up and blip-down shots acquired the complementary subsets of the k-space [3]. Multiband RF-excitations were combined with the blipped-CAIPI technique [4] to simultaneously acquire 2 slices per EPI-shot. The reconstruction of BUDA data used in-house developed MATLAB code to obtain distortion-free images.
(iii) Standard clinical brain MRI protocol: The protocol used for comparison was adapted from the clinical protocol used at MGH and included T1-weighted dark-fluid turbo spin echo (TSE), T2-weighted dark-fluid TSE, T2*-weighted FLASH, T2-weighted TSE, and diffusion-weighted single-shot EPI sequences.
RESULTS
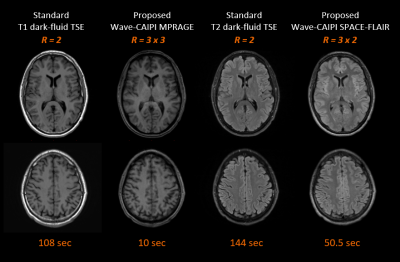
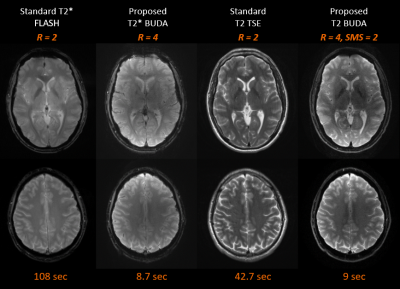
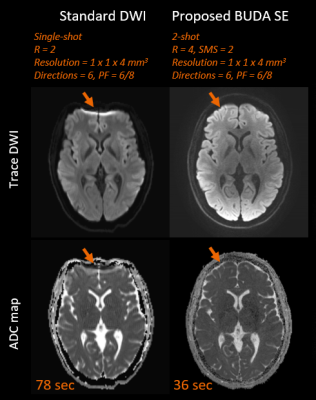
The total scan time was less than 2 minutes when using the proposed comprehensive distortion-free protocols. This includes T1-weighted MPRAGE (10 seconds + 2 seconds ACS data), T2-weighted dark-fluid SPACE-FLAIR (50.5 seconds), T2*-weighted images (8.7 seconds), T2-weighted images (9 seconds) and diffusion-weighted images (36 seconds). The image quality of T1-weighted MPRAGE and T2-weighted dark-fluid SPACE-FLAIR images was comparable to standard clinical reference in one volunteer (Figure 3). Figure 4 compares the image quality of distortion-free T2*-weighted, T2-weighted images and corresponding clinical standard. The diffusion-weighted images and clinical standard are shown in Figure 5.DISCUSSION
The imaging parameters for Wave-CAIPI and BUDA techniques were optimized to achieve similar spatial resolution and image contrast compared to the clinical protocol. One saturation band was applied to the proximal end of an imaging slab in both Wave-CAIPI MPRAGE and Wave-CAIPI SPACE-FLAIR to suppress signals close to the end edge of the imaging volume that would cause artifacts due to wrapping. Moreover, several techniques such as coil mixing [5] may further improve aliasing artifacts. This study introduced the possibility of acquiring axial thick-slice 2D-like MRI using 3D Wave-CAIPI techniques. It notes that wave corkscrews with large signal amplitudes during phase and partition encodings causes blurring artifacts when increasing the slice thickness. The BUDA method provided high in-plane resolution distortion-free images. As can be seen in Figure 5, the BUDA images had superior sharpness when compared to the clinical reference. The BUDA image appeared to be darker in the center, and this can be mitigated by pre-scan normalization. Additional investigation on volunteers and patients is warranted to further validate the benefits of our approach.CONCLUSION
A highly efficient distortion-free protocol combining the unique advantages of Wave-CAIPI and BUDA acquisition strategies provides comparable image quality, tissue contrast, and spatial resolution to standard clinical scans while keeping the total scan time to less than 2 minutes.Acknowledgements
No acknowledgement found.References
1. Polak, D. , Setsompop, K. , Cauley, S. F., Gagoski, B. A., Bhat, H. , Maier, F. , Bachert, P. , Wald, L. L. and Bilgic, B. (2018), Wave‐CAIPI for highly accelerated MP‐RAGE imaging. Magn. Reson. Med, 79: 401-406. doi:10.1002/mrm.26649
2. Polak, D. , Cauley, S. , Huang, S. Y., Longo, M. G., Conklin, J. , Bilgic, B. , Ohringer, N. , Raithel, E. , Bachert, P. , Wald, L. L. and Setsompop, K. (2019), Highly‐accelerated volumetric brain examination using optimized wave‐CAIPI encoding. J Magn Reson Imaging, 50: 961-974. doi:10.1002/jmri.26678
3. Liao, C. , Cao, X. , Cho, J. , Zhang, Z. , Setsompop K. , Bilgic, B. (2019), Highly efficient MRI through multi-shot echo planar imaging. In Wavelets and Sparsity XVIII (Vol. 11138, p. 1113818). International Society for Optics and Photonics. doi: 10.1117/12.2527183
4. Setsompop, K., Gagoski, B. A., Polimeni, J. R., Witzel, T., Wedeen, V. J., & Wald, L. L. (2012). Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magnetic resonance in medicine, 67(5), 1210–1224. doi:10.1002/mrm.23097
5. Cauley, S. , Polak D. , Liu W. , Bilgic B. , Gagoski B. , Grant E. P. , Conklin J. , Kirsch J. , Huang S. Y. , Setsompop K. , and Wald L. L. (2019), Geometric Coil Mixing (GCM) to Dampen Confounding Signals in MRI Reconstruction. Proceedings of ISMRM 27th.
Figures