0293
Lactate concentration from double quantum filtered (DQF) MRS in human breast tumour is associated with tumour grading and patient prognosis1Aberdeen Biomedical Imaging Centre, University of Aberdeen, Aberdeen, United Kingdom, 2Pathology Department, Aberdeen Royal Infirmary, Aberdeen, United Kingdom, 3Breast Unit, Aberdeen Royal Infirmary, Aberdeen, United Kingdom, 4Institute of Pharmacy and Biological Sciences, University of Strathclyde, Glasgow, United Kingdom
Synopsis
Upregulation of aerobic glycolysis and an elevated lactate accumulation have been linked to tumour aggressiveness. However, current evidence drawn from cell culture and small animal models remains controversial. Since lactate and lipid share the same spectral frequency, conventional MRS is inadequate in quantifying lactate under overwhelming lipid signal. Double quantum filtered (DQF) MRS allows excellent suppression of lipid signal from adipose breast tissues. We examined prognostic role of lactate concentration through a cross sectional study in grade II and III whole tumours freshly excised from patients with breast cancer using DQF MRS for the quantification of lactate concentration.
Introduction
Elevated lactate production is central to aerobic glycolysis of tumour metabolism, commonly known as the Warburg effect. The presence of lactate has been suggested to induce increased proliferative activity and in turn poor prognosis1. A higher concentration of lactate (> 9 mmol/L) has been found in metastatic orthotopically implanted mammary tumours in mice2,3 however opposite results were also observed using hyperpolarised 13C-pyruvate MRS4. Current controversy might arise from a predominant reliance on cell and animal models and a lack of patient studies. Since lactate and lipid share the same spectral frequency, conventional MRS is inadequate in extracting lactate in breast tissues. Double quantum filtered (DQF) MRS allows an efficient suppression of lipid5. We hypothesised that there is a difference in lactate concentration between high and low grade human breast cancer, as indicated by Warburg effect. To probe this hypothesis, we applied DQF MRS in whole tumours freshly excised from patients with breast cancer.Methods
Thirty female patients (age 39 – 78 years, 15 grade II and 15 grade III) with invasive ductal carcinoma participated in the study. Only patients undergoing breast surgery, with a tumour size larger than 1 cm on ultrasound were eligible. After tumour excision, the fresh specimen was immediately transported to the imaging centre for MRS scan. Once the scan was completed, the fresh specimen was transported to Pathology Department for formalin treatment and histopathological analysis, including lactate dehydrogenase A (LDH-A)6,7, Ki-678 and Nottingham Prognostic Index (NPI) (Figure 1). The study was approved by the North West – Greater Manchester East Research Ethics Committee (REC Ref: 16/NW/0032), and written informed consent was obtained prior to the MRS study.MRS Acquisition
All data were acquired on a 3.0 T whole body clinical MRI scanner (Achieva TX, Philips Healthcare, Best, Netherlands) using a body coil for uniform transmission and a 32-channel receiver coil for high sensitivity detection. Standard T1-weighted anatomical images were acquired with an isotropic voxel size of 1 mm, TR/TE of 5.2 ms/2.7 ms, encompassing the whole specimen. Lactate and reference spectra were collected from a single voxel snug-fit to the tumour. Lactate spectrum was obtained using DQF MRS5, with TR/TE of 1.25 s/144 ms, spectral editing frequency at 4.1 ppm and 512 averages. The reference spectrum was acquired from the same voxel with TR/TE of 1.25 s/144 ms, 16 averages.
Data Processing
All spectral data were pre-processed following standard procedures9 and quantified using AMARES algorithm10 in the jMRUI software11. Water and lactate amplitudes, with corresponding chemical shifts at 4.7 ppm and 1.3 ppm, were quantified from reference and lactate spectra respectively. Lactate concentration was then computed from lactate and water amplitudes accounting for literature breast tissue water composition12 and relaxation properties13.
Statistical Analysis
Statistical analysis was performed in SPSS software (Release 23.0, SPSS Inc., Chicago, IL, USA). Two-sample t-tests were carried out on patient characteristics and lactate concentration to assess group difference between grades, and Mann-Whitney U tests for non-normally distributed LDH-A, Ki-67 and NPI. Correlation analysis was conducted to determine the relationship between lactate concentration against LDH-A, NPI, Ki-67 and tumour size. The statistical results were classified as significant if p value was smaller than 0.05.
Results
The patient characteristics of the two groups are shown in Table 1, and there were no significant differences between groups, apart from NPI. There was a significantly higher (p = 0.0349, Table 2, Figure 2a) lactate concentration in grade III (7.7 ± 2.9 mM) than in grade II (5.5 ± 2.4 mM) breast tumours. There was a significantly higher (p = 0.0061) LDH-A expression in grade II (median: 300, interquartile range (IQR): 250 – 300) than in grade III (median: 100, IQR: 80 – 250) (Table 2, Figure 2b).There was a significant negative correlation between lactate concentration and LDH-A (r = -0.3734, p = 0.0421, Table 2, Figure 3a). There was a significant correlation between lactate concentration and NPI (r = 0.3618, p = 0.0495, Table 2, Figure 3b). There were no significant correlations between lactate concentration against Ki-67 expression (p = 0.1023, Table 2, Figure 3c) or tumour size (p = 0.3645, Figure 3d).
Discussion
We found that there was a significantly higher lactate concentration in grade III breast tumours compared to grade II, confirming Warburg effect in human breast cancer. Lactate concentration was negatively correlated with LDH-A, potentially due to feedback inhibition14 and depletion of substrates (pyruvate and nicotinamide adenine dinucleotide hydrogen)15 in higher grade tumour. Lactate concentration was correlated with NPI, indicating the potential value of lactate in patient prognosis. Lactate concentration was independent of proliferative marker Ki-67 and tumour size, indicating that lactate concentration is a sensitive biomarker specific to the underlying metabolic process. Our result is an important step in addressing the controversy around Warburg effect through lactate concentration observation from whole human breast tumour in a prospective cross sectional study.Conclusion
Our results show that lactate concentration is associated with tumour grading and patient prognosis, establishing Warburg effect and the prognostic value of lactate in breast cancer. This paves the way for potential clinical assessment of tumour aggressiveness and monitoring of disease progression and treatment effectiveness.Acknowledgements
The authors would like to thank Mr Nicholas Senn for conducting data auditing, Dr Matthew Clemence for clinical scientist support, Dr Tim Smith for biologist support, Ms Bolanle Brikinns for patient recruitment support, Ms Dawn Younie for logistic support, Prof Andrew M Blamire for advice on MRS. This project was funded by Friends of Aberdeen and North Centre for Haematology, Oncology and Radiotherapy (ANCHOR), and Sai Man Cheung was jointly supported by Elphinstone scholarship, Roland Sutton Academic Trust and John Mallard scholarship.References
1. Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013; 123(9): 3685–3692.
2. Serganova I, Rizwan A, Ni X, et al. Metabolic Imaging: A Link between Lactate Dehydrogenase A, Lactate, and Tumor Phenotype. Clin Cancer Res. 2011; 17(19): 6250–6261.
3. Rizwan, A, Serganova, I, Khanin, R, et al. Relationships between LDH-A, lactate, and metastases in 4T1 breast tumors. Clin Cancer Res. 2013; 19(18): 5158 – 5169.
4. Xu HN, Kadlececk S, Profka H, et al. Is higher lactate an indicator of tumor metastatic risk? A pilot MRS study using hyperpolarized 13C-Pyruvate. Acad Radiol. 2014; 21(2): 223–231.
5. He, Q, Shungu, DC, van Zijl, PCM, et al. Single-scan in vivo lactate editing with complete lipid and water suppression by selective multiple-quantum-coherence transfer (Sel-MQC) with application to tumors. J Magn Reson, Series B. 1995; 106(3): 203 – 211.
6. Dong, T, et al. Tumor LDH-A expression and serum LDH status are two metabolic predictors for triple negative breast cancer brain metastasis. Sci Rep. (2017); 7: 6069.
7. McClelland, RA, et al. Automated Quantitation of Immunocytochemically Localized Estrogen Receptors in Human Breast Cancer. Cancer Research. (1990); 50: 3545–3550.
8. Tuominen, VJ, Ruotoistenmäki, S, Viitanen, A, Jumppanen, M & Isola, J. ImmunoRatio: a publicly available web application for quantitative image analysis of estrogen receptor (ER), progesterone receptor (PR), and Ki-67. Breast Cancer Res. (2010); 12: R56.
9. Baik H-M, Su M-Y, Yu H, Nalcioglu O, Mehta R. Quantification of Choline-containing Compounds in Malignant Breast Tumors by 1H MR Spectroscopy Using Water as an Internal Reference at 1.5 T. MAGMA. 2006;19(2):96-104.
10. Vanhamme L, van den Boogaart A. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997; 129(1): 35–43.
11. Naressi A, Couturier C, Castang I, et al. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med. 2001; 31(4): 269–286.
12. Sijens, PE, Dorrius, MD, Kappert, P, et al. Quantitative multivoxel proton chemical shift imaging of the breast. Magn Reson Imag. 2010; 28(3): 314 – 319.
13. Annarao, S, Thomas, K, Pillarsetty, N, et al. In vivo lactate T1 and T2 relaxation measurements in ER-positive breast tumours using SS-SelMQC editing sequence. Proceedings of the 19th Annual International Society of Magnetic Resonance in Medicine (ISMRM), Montréal, Québec. 2011; p.3158.
14. Valvona, CJ, Fillmore, HL, Nunn, PB, Pilkington, GJ. The regulation and function of lactate dehydrogenase A: Therapeutic potential in brain tumour. Brain Pathology. (2016); 26(1): 3–17.
15. Spriet, LL, Howlett, RA, Heigenhauser, GJF. An enzymatic approach to lactate production in human skeletal muscle during exercise. Med Sci Sports Exerc. (2000); 32(4): 756 – 763.
Figures
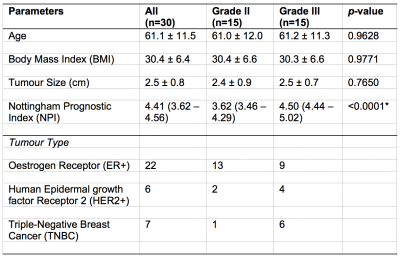
Table 1. Patient demographics.
Patient demographics and routine histopathological findings of excised breast tumours are shown for each group and the entire cohort. Quantitative data were expressed as mean and standard deviation, while qualitative data expressed as number of positive cases. Significant differences are marked by ‘*’.
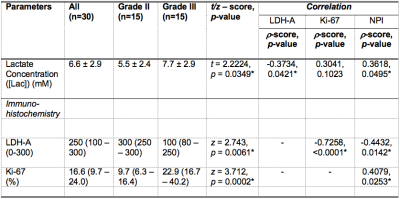
Table 2. A summary of statistical findings in this study.
Lactate concentration, lactate dehydrogenase A (LDH-A) expression and proliferative marker Ki-67 expression are shown for groups and the entire cohort. Correlation (Spearman’s rho (ρ)) scores of lactate concentration against LDH-A, Ki-67 and Nottingham Prognostic Index (NPI) are also shown. There was a significant higher lactate concentration in grade III breast tumour compared to grade II. Lactate concentration was significantly correlated with NPI.
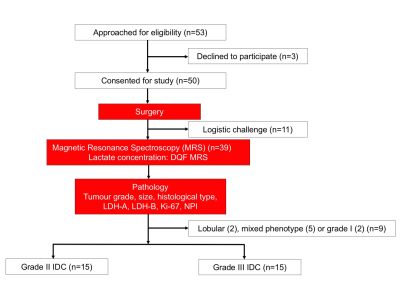
Figure 1. Study design.
A two-group cross sectional arrangement in a flow chart. Patients underwent wide local excision or mastectomy, and the freshly excised tumours were scanned on a 3.0 T clinical MRI scanner to derive lactate concentration of the whole tumour using double quantum filtered (DQF) MRS. Histopathological analysis was carried out to derive the tumour grade, size, lactate dehydrogenase A and B, proliferative marker Ki-67 and Nottingham Prognostic Index (NPI). Thirty patients with invasive ductal carcinoma (IDC) (15 grade II and 15 grade III) participated in the study.
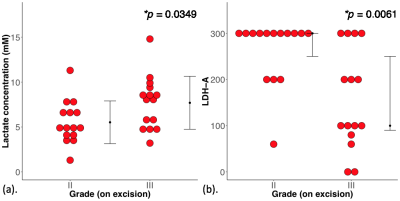
Figure 2. Group difference results.
The group difference in (a) lactate concentration and (b) lactate dehydrogenase A (LDH-A) expression shown in dot plots. Each dot represents the measurement obtained in each patient, and the dots are organised in two columns corresponding to the tumour grades. The t-test was performed between the groups for lactate concentration, while Mann-Whitney U test for LDH-A. There is a significantly higher lactate concentration in grade III breast tumour compared to grade II. There is a significantly lower LDH-A expression in grade III compared to grade II.
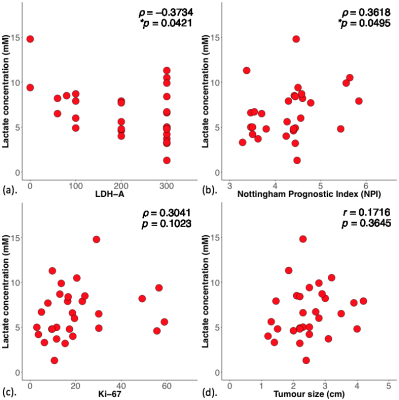
Figure 3. Correlation results.
The correlation of lactate concentration with (a) lactate dehydrogenase A (LDH-A) expression, (b) Nottingham Prognostic Index (NPI), (c) Ki-67 expression and (d) tumour size within the entire cohort are shown in scatter plots. The corresponding Spearman’s rank correlation ρ score (a-c), Pearson’s correlation r score (d) and p value are displayed. There is significant negative correlation between lactate concentration and LDH-A expression. A significant correlation is observed between lactate concentration and NPI.