0292
Evaluating the sensitivity of ADC to childhood neuroblastoma pathology in vivo using Gaussian mixture modeling and computational pathology1Radiotherapy and Imaging, Institute of Cancer Research, London, Sutton, United Kingdom, 2Clinical studies, Institute of Cancer Research, London, Sutton, United Kingdom, 3Molecular pathology, Institute of Cancer Research, London, Sutton, United Kingdom
Synopsis
We use Gaussian mixture modeling, computational pathology and MRI-histopathology registered datasets to evaluate i) the sensitivity of diffusion-weighted imaging to the rich histopathology of childhood neuroblastoma and ii) the sensitivity of the derived apparent diffusion coefficient, ADC, as a biomarker of response to a potent MYCN-targeted small molecule inhibitor in the Th-MYCN mouse, a faithful model of high-risk MYCN-driven disease.
Introduction
The clinical outcome of children with high-risk neuroblastoma, a cancer of the sympathetic nervous system, remains poor.1 The oncogene MYCN plays a central role in the biology of high-risk neuroblastoma. Small molecule inhibitors targeting the stability of the MYCN protein have shown strong anti-tumor activity, via the induction of apoptosis in the Th-MYCN transgenic mouse model of neuroblastoma. Selective inhibitors of mTOR activity, such as vistusertib, which induce MYCN phosphorylation necessary to trigger its proteasomal degradation are currently being evaluated in early-phase pediatric clinical trials. Imaging biomarkers of response would be a valuable addition to these trials especially since conventional pharmacodynamic biomarkers are difficult to evaluate in children. Diffusion-weighted MRI (DWI) is increasingly being used for assisting neuroblastoma diagnosis.2Purpose
The aims of this study were to evaluate whether the apparent diffusion coefficient (ADC) could provide a biomarker of response to vistusertib in the Th-MYCN mouse and to decipher the pathological determinant(s) contributing to global and regional variations in intratumor ADC using computational pathology and Gaussian mixture models (GMMs).Methods
MRI: Tumor-bearing Th-MYCN mice were imaged at 7T, prior to and 24 hours after treatment with 25mg/kg vistusertib (AstraZeneca, n=14) or vehicle control (n=12). Diffusion-weighted imaging was performed using an EPI readout (5 b-values=200-1000 s.mm-2, TE=32ms, TR=1500ms, NS=4, FOV=3x3cm, 128x128 matrix, 1mm slice-thickness). ADC was calculated voxel-wise using a robust Bayesian approach.3Computational Pathology: Guided by T2w-MRI, tumors (ntreated=7, nvehicle=11) were carefully excised and orientated for histopathological processing. Formalin-fixed and paraffin-embedded tumors were sectioned (3μm). Hematoxylin and eosin (H&E)-stained whole-slide images were digitized (20x magnification, 0.46μm pixel resolution, Hamamatsu NanoZoomer-XR) and analysed using CRImage (Bioconductor).4 Cells were automatically segmented and classified into 5 categories: undifferentiated neuroblasts, differentiating neuroblasts, apoptotic cells, lymphocytes, stromal cells. Density maps of segmented cells and classified undifferentiated, apoptotic and differentiating neuroblasts matching the MRI resolution were generated and automatically registered as previously described.5,6
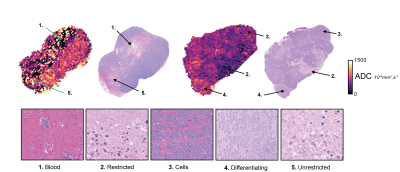
Gaussian mixture modeling for tumor clustering: From visual inspection of the registered ADC & H&E images, we identified 5 pathological determinants of regional heterogeneity on ADC maps (Figure 1). We used GMMs to cluster ADC values, using Bayesian and Akaike information criteria (BIC, AIC) to determine the appropriate number of clusters (2-10 were tested). GMMs were applied in 6 tumours from the vehicle-control group, containing all the areas of interest. A GMM was applied 1000 times on the ADC data with random initialisations and the median value of the threshold for each cluster was selected. All tumors were classified using the defined clusters.
Results
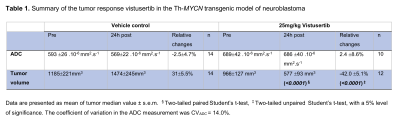
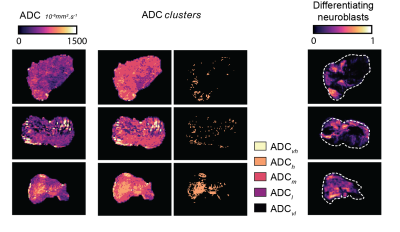
Treatment with vistusertib was associated with significantly lower fraction of undifferentiated neuroblasts (66±4% vs 21±3%, p<0.0001) and higher fraction in apoptotic cells (17±3% vs 57±3%, p<0.0001) at 24h compared to vehicle control and a significant reduction in tumor burden in the Th-MYCN model (Table 1). There was no significant change in tumor ADC over 24h treatment with vistusertib.BIC and AIC criteria suggested using between 3 to 8 clusters, which we fixed to 5 based on our initial hypothesis and which was corroborated by the sub-regional analysis (Figures 1 & 2.A). The 5 compartments were categorised as: ADCvl (0-188.10-6 mm2.s-1), ADCl (188-452.10-6 mm2.s-1), ADCm (452-850.10-6 mm2.s-1), ADCh (850-1400.10-6 mm2.s-1) and ADCvh (1400-2000.10-6 mm2.s-1). ADCm and ADCh contained higher density of undifferentiated neuroblasts than the rest of the tumor. In the treated tumors, areas of high density of apoptotic neuroblasts corresponded to both low ADC (ADCvl and ADCl) and higher ADC values and compartments (Figure 2.B).
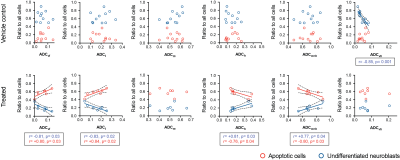
Quantitative analysis (Figure 3) showed that the fraction of apoptotic cells in treated tumors positively correlated with the fraction of pixels belonging to the ADCvl and ADCl compartments (hemorrhage and restricted diffusion) and inversely correlated with the fraction of pixels belonging to the ADCm+h compartments (typically richer in undifferentiated cells). Additionally, the ADCh compartment was sensitive to regions rich in differentiating neuroblasts in three differentiating tumors (Figure 4).
Discussion
Our study demonstrates the sensitivity of DWI to the rich histopathology of neuroblastoma but reinforces the caution raised by other studies regarding the sensitivity of median tumor ADC as a biomarker of tumor response to therapy.7 Factors affecting the ADC response are the small amount of extracellular space (high-risk neuroblastoma is stroma-poor), diffuse apoptosis, inter-tumor heterogeneity and the presence of coagulative (low ADC) and liquefactive (high ADC) tissue damage. We used our insights of neuroblastoma histology to guide GMM clustering to show that i) the increase in apoptosis and extended tissue damage following treatment actually correlated with regions where water diffusion is restricted (low ADC) and ii) the sensitivity of DWI to differentiation used clinically to discriminate stroma-rich differentiating benign form of neuroblastoma from stroma-poor aggressive neuroblastoma could potentially be extended to detect differentiating regions within stroma-poor aggressive neuroblastoma.2Conclusion
Global tumor ADC is not a sensitive imaging biomarker of tumor response to widespread apoptosis induced by vistusertib in the Th-MYCN model of neuroblastoma. Based on our insights of neuroblastoma histopathology, we guided the application of GMMs to derive ADC compartments and demonstrated the sensitivity of low ADC values for treatment-induced apoptosis and the sensitivity of higher ADC values to regions rich in differentiating neuroblasts.Acknowledgements
The Institute of Cancer Research Cancer Research UK Cancer Imaging Centre in association with the MRC and Department of Health grant C1060/A10334, Rosetrees Trust, Children with Cancer UK, Cancer Research UK grant C16412/A27725.References
1. Matthay KK, Maris JM, Schleiermacher G et al. Neuroblastoma. Nat Rev Dis Primers. 2016;2:16078.
2. Wen Y, Peng Y, Duan XM et al. Role of diffusion-weighted imaging in distinguishing thoracoabdominal neuroblastic tumours of various histological types and differentiation grades. J Med Imaging Radiat Oncol. 2017;61(6):718-724.
3. Walker-Samuel S, Orton M, Boult JKR et al. Improving apparent diffusion coefficient estimates and elucidating tumor heterogeneity using Bayesian adaptive smoothing.
4. Pau G, Fuchs F, Sklyar O, et al. EBImage - an R package for image processing with applications to cellular phenotypes. Bioinformatics. 2010;26:979-981
5. Zormpas-Petridis K, Blackledge MD, Clarke M et al. T1 mapping of neuroblastoma pathology: insight from a computational pathology study in the Th-MYCN transgenic mouse model. Proc Int Soc Magn Res Med. 2019;0584.
6. Zormpas-Petridis K, Jerome NP, Blackledge MD et al. MRI Imaging of the Hemodynamic Vasculature of Neuroblastoma Predicts Response to Anti-angiogenic Treatment. Cancer Res. 2019;79:2978-91.
7. Sinkus R, Van Beers BE, Vilgrain V et al. Apparent diffusion coefficient from magnetic resonance imaging as a biomarker in oncology drug development. Eur. J. Cancer. 2019;48:425-431.
Figures