0290
Progressive site-directed therapy for oligo-progressive castration-resistant prostate cancer1Urology, Tokyo Medical and Dental University, Tokyo, Japan, 2Biomedical Engineering, Tokai University School of Engineering, Kanagawa, Japan, 3Radiology, Keio University School of Medicine, Tokyo, Japan, 4Radiology, Advanced Imaging Center, Yaesu Clinic, Tokyo, Japan, 5Radiation Therapeutics and Oncology, Tokyo Medical and Dental University, Tokyo, Japan, 6PixSpace, Ltd., Fukuoka, Japan
Synopsis
Locoregional therapy for oligometastatic prostate cancer has generated great interest. However, the benefit for castration-resistant prostate cancer (CRPC) has not been fully demonstrated. According to the current study, whole body-MRI incorporating DWI identified a substantial number of oligo-progressive CRPC patients (OP-CRPC) with a number of progressive lesions 3 or less. Progressive site-directed therapy (PSDT) to OP-CRPC provided a high treatment effect in terms of prostate-specific antigen (PSA) response, especially for patients with longer PSA-doubling time. Furthermore, this study identified the site-dependencies of the PSDT treatment effect; patients whose progressive site was localized in the pelvis were good candidates for PSDT.
Introduction
Castration-resistant prostate cancer (CRPC) is considered to be fatal, with the median survival ranging from 9 to 30 months.1 New treatment strategies for CRPC must be investigated, and locoregional therapy against metastatic prostate cancer has generated great interest.2-4 This treatment strategy includes prostate-directed therapy and metastasis-directed therapy. The utility of metastasis-directed therapy was shown in a prospective randomized phase 2 trial.5 For patients with biochemical recurrence and three or fewer metastatic lesions after primary prostate cancer treatment, metastasis-directed therapy improved biochemical recurrence-free survival. These findings suggest that locoregional therapy should be considered in prostate cancer with low metastatic burden. However, the survival benefit of locoregional therapy for CRPC has not been fully demonstrated. In this study, we evaluated treatment outcomes of locoregional radiotherapy based on the progressive site of CRPC.Patients and Methods
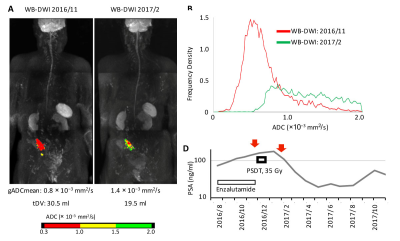
This was a retrospective single-institution analysis, which was approved by the institutional review board (study number: M2016-170). We reviewed 101 consecutive CRPC patients who underwent whole-body MRI (WB-MRI) before starting a new line of anti-cancer therapy between 2014 and 2018. WB-MRI incorporating DWI (WB-DWI) was performed by using a 1.5-T MR scanner (Philips Ingenia 1.5T). A single-shot echo-planar imaging sequence with a short inversion time inversion recovery pre-pulse for fat suppression was used for the background body signal suppression (DWIBS) sequence (b-value of 999 s/mm2).6,7 A single radiologist with 12 years’ experience in body DWI reviewed the WB-DWI results in accordance with the MET-RADS-P criteria8; oligoprogressive CRPC (OP-CRPC) patients were defined as CRPC patients with 3 or less progressive lesions. Tumor activity was evaluated based on DWIBS signal. Computed DWI of the prostate with a b-value of 2000 sec/mm2 was performed when needed.9,10 The mean value of the whole-body global apparent-diffusion coefficient value (gADCmean) and tumor diffusion volume (tDV), which represents all pixels in the progressive lesions showing an ADC value from 0.3 to 2.0 × 10−3 mm2/s, were calculated using Attractive BDScore (PixSpace, Ltd, Fukuoka, Japan). Patients with OP-CRPC on WB-DWI were considered for progressive site-directed therapy (PSDT) of all detected progressive lesions. Systemic therapy regimens were continued unchanged in accordance with the institutional treatment protocol. PSDT was performed with the intent to target all progressive sites with radiotherapy. External beam radiotherapy using 60-78 Gy (2 Gy per fraction) to the prostate/lymph node metastasis and 30-39 Gy (2-3 Gy per fraction) to the bone metastasis was applied (Fig. 1, 2). Time to prostate-specific antigen (PSA) progression after PSDT was calculated from the start of radiotherapy to PSA progression (PSA nadir + 2 ng/ml).11Results
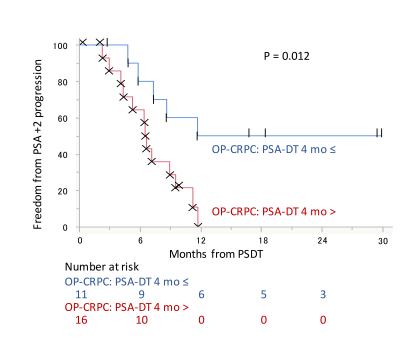
Of the 115 eligible CRPC patients, 45 (39%) were diagnosed with OP-CRPC. Of these 45 OP-CRPC, 27 (60%) underwent PSDT without changing systemic therapy. The median time from WB-DWI to PSDT was 1.6 months (range: 1-7 months). The most frequent site of PSDT was the pelvic bone (n = 11), followed by the prostate (n = 7). In total, 21 (89%) patients treated with PSDT had a decline in PSA. A decrease in PSA levels of at least 50% in response to PSDT (50% PSA-decline) was observed in 17 (67%) patients (Fig. 3). Of the 27 patients treated with PSDT, 18 (67%) experienced PSA progression, and their median time to PSA progression was 6.6 months (range: 2.3-11.8 months). The median follow-up of the remaining 9 patients who did not experience PSA progression was 16.8 months (0.3-29.9 months). Early toxicity of PSDT was observed in one (2%) patient (proctitis, RTOG grade 2). Of the potential prognostic variables, PSA-doubling time (PSA-DT) was the significant predictor for time to PSA progression (hazard ratio (HR) 1.3; P = 0.0045), as well as targeted site localization (HR 3.8; P = 0.036). The PSA-progression-free survival (PSA-PFS) was significantly longer in the OP-CRPC patients with a PSA-DT ≥ 4 months compared with those with a PSA-DT < 4 months. (median PSA-PFS: 11.7 vs 6.6 months, P = 0.012; Fig. 4). The PSA-PFS was significantly longer in the pelvic OP-CRPC group compared with the non-pelvic OP-CRPC group (median time to PSA-PFS: 9.0 vs. 4.8 months, P = 0.017; Fig. 5). No significant difference was observed in terms tDV, gADCmean or other possible prognostic factors.Discussion
PSDT achieves PSA response in the majority of OP-CRPC patients, which delays intensification of systemic therapy, especially for patients with longer PSA-DT. Furthermore, the absence of progressive disease spread outside of the pelvis was identified as a good prognostic factor after PSDT. The spread of progressive lesions was an important factor when we consider whether to administer PSDT to OP-CRPC patients. Extensive metastatic CRPC is known to have poor prognosis, and a low ADC value might be a predictor of radioresistance.12 However, in the current study, tDV and gADCmean had no significant impact on the PSA-PFS.Conclusion
WB-DWI is useful to identify the progressive lesion to target in CRPC patients, and PSDT to OP-CRPC provided a high treatment effect in terms of PSA response. The patients whose progressive site was localized in the pelvis, and PSA-DT was longer than 4 months were good candidates for PSDT.Acknowledgements
NoneReferences
[1] Kirby M, Hirst C Crawford ED. Characterising the castration-resistant prostate cancer population: A systematic review. Int J Clin Pract. 2011; 65: 1180-1192.
[2] Leyh-Bannurah SR, Gazdovich S, Budaus L, et al. Local therapy improves survival in metastatic prostate cancer. Eur Urol. 2017; 72: 118-124.
[3] Ost P, Jereczek-Fossa BA, As NV, et al. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: A multi-institutional analysis. Eur Urol. 2016; 69: 9-12.
[4] Ricardi U, Badellino S Filippi AR. Clinical applications of stereotactic radiation therapy for oligometastatic cancer patients: A disease-oriented approach. J Radiat Res. 2016.
[5] Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase ii trial. J Clin Oncol. 2018;36:446-453.
[6] Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. 2004; 22: 275-82.
[7] Kwee TC, Takahara T, Ochiai R, et al. Whole-body diffusion-weighted magnetic resonance imaging. Eur J Radiol. 2009; 70: 409-17.
[8] Padhani AR, Lecouvet FE, Tunariu N, et al. Metastasis reporting and data system for prostate cancer: Practical guidelines for acquisition, interpretation, and reporting of whole-body magnetic resonance imaging-based evaluations of multiorgan involvement in advanced prostate cancer. Eur Urol. 2017; 71: 81-92.
[9] Waseda Y, Yoshida S, Takahara T, et al. Utility of computed diffusion-weighted mri for predicting aggressiveness of prostate cancer. JMRI. 2017; 46: 490-496.
[10] Ueno Y, Takahashi S, Ohno Y, et al. Computed diffusion-weighted mri for prostate cancer detection: The influence of the combinations of b-values. Br J Radiol. 2015; 88: 20140738.
[11] Lohaus F, Zophel K, Lock S, et al. Can local ablative radiotherapy revert castration-resistant prostate cancer to an earlier stage of disease? Eur Urol. 2019; 75: 548-551.
[12] Padhani AR, Liu G, Koh DM, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia. 2009; 11: 102-125.
Figures