0289
Utility of computed diffusion-weighted imaging for evaluating primary prostate cancer in whole-body MRI1Department of Radiology, Keio University School of Medicine, Tokyo, Japan, 2Department of Radiology, Advanced Imaging Center Yaesu Clinic, Tokyo, Japan, 3Department of Urology, Tokyo Medical and Dental University Graduate School, Tokyo, Japan, 4Department of Biomedical engineering, Tokai University School of Engineering, Kanagawa, Japan, 5Department of Radiology, Nuclear Medicine and Molecular Imaging, University Medical Center Groningen, Groningen, Netherlands, 6Department of Biostatistics Unit, Clinical and Translational Research Center, Keio University Hospital, Tokyo, Japan
Synopsis
The purpose of this study is to determine the value of applying computed DWI to a whole-body MRI/DWI protocol to acquire computed high b-value (2000 s/mm2) diffusion-weighted images for local prostate cancer evaluation. Based on the results, computed diffusion-weighted images obtained from whole-body MRI provide a similar diagnostic performance compared to pelvic bi-parametric MRI for the detection of primary prostate cancer. Computed DWI is a straightforward postprocessing technique without the need for additional image acquisition time. It can be recommended for use in routine clinical practice in whole-body MRI protocols for concurrent evaluation of primary and metastatic prostate cancer.
Introduction
Since recently, there has been an increasing interest in the utility of locoregional therapy for metastatic prostate cancer (PCa).1-4 This treatment paradigm shift requires a robust imaging technique to evaluate active disease in both metastatic sites throughout the body and the primary tumor location in the prostate.Whole-body (WB) MRI/diffusion-weighted imaging (DWI) is a one-step examination, which allows evaluating extra-osseous and osseous lesions, without involving any radiation exposure.5 Nevertheless, in order not to prolong scan time, a standard WB-DWI protocol usually employs relatively low b-values of 0 and 1000 s/mm2, which may be suboptimal for primary PCa evaluation. Computed DWI is a computational technique that synthesizes DWI at any arbitrary (high) b-value from a DWI dataset that has been acquired with at least two different (lower) b-values.6 By using this technique, disadvantages associated with direct, native high b-value DWI acquisitions such as poor signal-to-noise ratio and image distortion, may be circumvented.7 Moreover, computed DWI require no additional acquisition time and can be generated automatically by the MRI software at the time of image acquisition.
Our study aimed to determine the value of applying computed DWI to a WB-MRI/DWI protocol to acquire computed high b-value (2000 s/mm2) DWI for local PCa evaluation.
Patients and Methods
Patients who underwent pelvic bi-parametric MRI (bpMRI) including T2-weighted imaging (T2WI) and DWI for PCa screening and subsequent WB-MRI for staging under the diagnosis of PCa, were prospectively included between September 2014 and September 2017. WB-MRI was performed from skull base to midthighs with a 1.5 or 3.0-T system (Philips, Intera Achieva 1.5-T/Ingenia 3.0-T, Philips Healthcare, Best, the Netherlands) (Figure 1). The WB-MRI protocol included diffusion-weighted whole-body imaging with background body signal suppression (DWIBS)8 and T2WI. Computed DWI of the prostate with a b-value of 2000 s/mm2 (computed DWI2000) was generated from lower b-value DWI (0 and 1000 s/mm2) from WB-MRI, based on a standard monoexponential fit model. Applied sequence parameters are shown in Figure 2.Two radiologists used the PI-RADS (version 2) to assess the diagnostic performances of the following three bpMRI data sets for the detection of clinically significant PCa (Gleason group ≥2): (a) pDWI2000 + pT2WI (both from pelvic bpMRI), (b) native DWI1000 + T2WI (both from WB-MRI), (c) computed DWI2000 and T2WI (both from WB-MRI). Systemic or transrectal-ultrasound-targeted biopsy results were used as reference standard. ROC analysis based on logistic regression was performed to assess the diagnostic value of the three imaging bpMRI data sets for clinically significant PCa. The optimal cut-off points for maximum accuracy were determined using the Youden index. AUCs were compared with the two-variable chi-squared test.
Results
Fifty-one patients were included. The ROC curves of three bpMRI data sets for clinically significant PCa are shown in Figure 3. The AUCs of the three bpMRI data sets were as follows: 0.89/0.87 for pDWI2000 + pT2WI; 0.86/0.81 for computed DWI2000 + T2WI; and 0.64/0.61 for native DWI1000 + T2WI for the two different readers, respectively. There were significant differences between pDWI2000 + pT2WI and native DWI1000 + T2WI (P < 0.001, for both readers), and between that with computed DWI2000 + T2WI and native DWI1000 + T2WI (P < 0.001, for both readers). No significant difference in diagnostic performance was found between pDWI2000 + pT2WI and computed DWI2000 + T2WI.The sensitivity/specificity/accuracy (in %) for clinically significant PCa with an optimal cut-off of PI-RADS score 3 were 92.2/70.9/78.9 for pDWI2000 + pT2WI, 90.9/66.9/76.0 for computed DWI2000 + T2WI, 68.8/47.2/55.4 for native DWI1000 + T2WI in reader 1, and 89.6/69.3/77.0 for pDWI2000 + pT2WI, 87.0/65.4/73.5 for computed DWI2000 + T2WI, 67.5/46.5/54.4 for native DWI1000 + T2WI in reader 2. Representative cases of PCa in the transitional zone and peripheral zone are shown in Figures 4 and 5, respectively.
Discussion
Based on the results, computed DWI2000 which requires no additional acquisition time provided comparable diagnostic performance to pDWI2000, and superior performance to native DWI1000 in this study. Therefore, a standard incorporation of computed DWI2000 into standard clinical WB-MRI protocols for PCa may be worthwhile. As shown in this study, WB-MRI with computed DWI would enable us to evaluate the primary prostate lesion more accurately. Therefore, computed DWI with WB-MRI has a potential usefulness for evaluating locoregional therapy by detecting both metastatic lesions and visualizing the PCa primary site. Furthermore, compared to prostate-specific membrane antigen positron-emission tomography, which may also be useful for whole-body tumor viability assessment,9-11 WB-MRI is less expensive and does not involve any radiation exposure, as a result of which it is more amenable for frequent follow-up imaging.Our study had several limitations. First, studies in the therapy response assessment setting with computed DWI are desirable. Second, the sample size was relatively small from a single institution. Further larger, multicenter validation studies are necessary to confirm the results of this study.
Conclusion
Computed DWI derived from WB-MRI provides a similar diagnostic performance compared to pelvic bpMRI for primary PCa detection. Since computed DWI is a straightforward postprocessing technique that requires no additional image acquisition time, it can be recommended for routine implementation in WB-MRI protocols for simultaneous primary and metastatic PCa evaluation.Acknowledgements
The authors thank Mr. Mizuki Akatsuka helping us collect the data.References
1. Leyh-Bannurah SR, Gazdovich S, Budäus L, et al. Local therapy improves survival in metastatic prostate cancer. Eur Urol. 2017;72:118-124
2. Ost P, Jereczek-Fossa BA, As NV, et al. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: a multi-institutional analysis. Eur Urol. 2016; 69:9-12
3. Ricardi U, Badellino S, Filippi AR. Clinical applications of stereotactic radiation therapy for oligometastatic cancer patients: a disease-oriented approach. J Radiat Res. 2016;57:i58-i68
4. Yoshida S, Takahara T, Arita Y, et al. Progressive site-directed therapy for castration-resistant prostate cancer: localization of the progressive site as a prognostic factor. Int J Radiat Oncol Biol Phys. 2019;S0360-3016(19)30837-5
5. Lecouvet FE, Talbot JN, Messiou C, et al. Monitoring the response of bone metastases to treatment with Magnetic Resonance Imaging and nuclear medicine techniques: a review and position statement by the European Organisation for Research and Treatment of Cancer imaging group. Eur J Cancer. 2014;50:2519-2531
6. Blackledge MD, Leach MO, Collins DJ, Koh DM. Computed diffusion-weighted MR imaging may improve tumor detection. Radiology. 2011;261:573-581
7. Rosenkrantz AB, Chandarana H, Hindman N, et al. Computed diffusion-weighted imaging of the prostate at 3 T: impact on image quality and tumour detection. Eur Radiol. 2013;23:3170-3177
8. Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, van Cauteren M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. 2004;22:275-282
9. Kranzbühler B, Müller J, Becker AS, et al. Detection rate and localization of prostate cancer recurrence using 68Ga-PSMA-11 PET/MRI in patients with low PSA values ≤ 0.5 ng/ml. J Nucl Med. 2019;pii: jnumed.118.225276
10. Zhou J, Gou Z, Wu R, Yuan Y, Yu G, Zhao Y. Comparison of PSMA-PET/CT, choline-PET/CT, NaF-PET/CT, MRI, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a systematic review and meta-analysis. Skeletal Radiol. 2019;DOI:10.1007/s00256-019-03230-z
11. Perera M, Papa N, Roberts M, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. 2019;pii: S0302-2838(19)30095-8
Figures
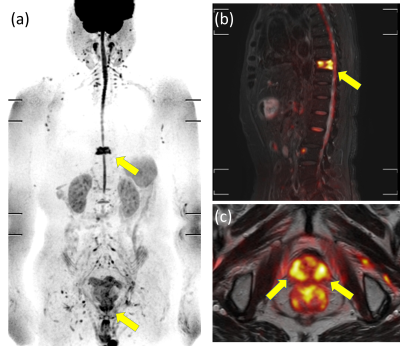
Figure 1. An 82-year-old man with adenocarcinoma (cT3aN0M1b, Grade group 4, PSA 24.9 ng/mL). (a) Maximum intensity projection of native DWIBS at a b-value of 1000 s/mm2, (b) sagittal fusion image of STIR + native DWIBS at a b-value of 1000 s/mm2, and (c) axial fusion image of T2WI + native DWIBS at a b-value of 1000 s/mm2 show an abnormally high signal intensity focus in the ninth thoracic vertebra and bilateral peripheral zones of the prostate (arrows), corresponding to a vertebral metastasis and primary prostate cancer lesions, respectively.
PSA = prostate-specific antigen.
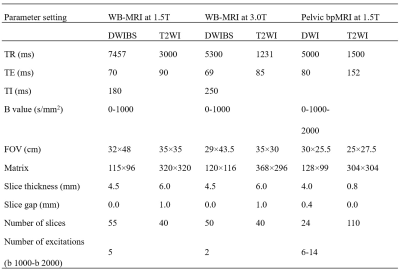
Figure 2. MRI protocol for pelvic bpMRI and WB-MRI at 1.5 and 3.0-T.
WB-MRI = whole-body MRI, bpMRI = bi-parametric MRI, DWIBS = diffusion-weighted whole-body imaging with background body signal suppression, T2WI = T2-weighted imaging, TR = repetition time, TE = echo time, TI = inversion time, FOV = field of view.

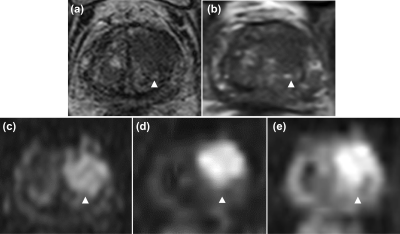
Figure 4. Representative case of a 21-mm adenocarcinoma in the transitional zone of the left lobe in an 81-year-old man (cT3a, Grade group 4, PSA 4.56 ng/mL). (a), (b) Axial T2WI from conventional pelvic MRI and whole-body MRI/DWI show a low signal intensity focus, respectively (arrowhead). (c) Pelvic DWI2000, (d) computed DWI2000 from whole-body MRI/DWI, and (e) native DWI1000 from whole-body MRI/DWI show a high signal intensity focus (arrowhead), corresponding to a PI-RADS v2 score of 5.
PSA = prostate-specific antigen.
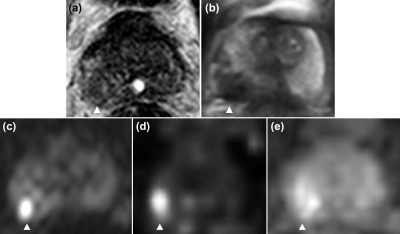
Figure 5. Representative case of a 10-mm adenocarcinoma in the peripheral zone of the right lobe in a 71-year-old man (cT2a, Grade group 4, PSA 30.6 ng/mL). (a), (b) Axial T2WI from conventional pelvic MRI and whole-body MRI/DWI, (c) pelvic DWI2000, (d) computed DWI2000 from whole-body MRI/DWI show a abnormal signal intensity focus, respectively (arrowhead), corresponding to a PI-RADS v2 score of 4. (e) Native DWI1000 from whole-body MRI/DWI shows an amibiguously increased signal intensity focus (arrowhead).
PSA = prostate-specific antigen.