0288
Extracellular pH Changes Induced by Immuno-Thermal Ablation in a Murine Colorectal Cancer Model1Radiology & Biomedical Imaging, Division of Bioimaging Sciences, Yale University School of Medicine, New Haven, CT, United States, 2Radiology & Biomedical Imaging, Section of Interventional Radiology, Yale University School of Medicine, New Haven, CT, United States, 3University of Connecticut School of Medicine, Farmington, CT, United States, 4Department of Biomedical Engineering, Yale University School of Medicine, New Haven, CT, United States, 5Yale Cancer Center, Yale University School of Medicine, New Haven, CT, United States, 6Department of Internal Medicine, Section of Medical Oncology, Yale University School of Medicine, New Haven, CT, United States
Synopsis
Acidification of tumor microenvironment is associated with aggressive tumor growth and facilitate resistance to anti-cancer therapies. Extracellular pH (pHe) mapping with BIRDS is used to differentiate ablated and non-ablated tumors in the setting of systemic immunotherapy of murine colorectal cancer. Combination of Cryoablation with Dual Immune Checkpoint Blockade (DICB) resulted in a significant pHe increase compared to control tumors. This work demonstrates the feasibility of measuring pHe with BIRDS in a murine colorectal cancer model. pHe imaging could serve as a non-invasive imaging biomarker for tumor microenvironment assessment and monitoring of metabolic changes after immuno-thermal ablation therapy.
Introduction:
Colorectal cancer (CRC) is the third most common malignancy in the world, the fourth most commonly diagnosed cancer and the second leading cause of cancer-related death in the United States1. Cancer cells possess a hyper-glycolytic metabolism even in the presence of sufficient oxygen (“Warburg effect”). The result is the synthesis of large amounts of lactate and hydrogen ions (i.e., protons) and their transfer into the extracellular space. The consequent acidification of the tumor microenvironment due to the output of protons and lactate is associated with aggressive tumor growth and potentially helps facilitate resistance to anti-cancer therapies, e.g. by promoting immunoevasive mechanisms. Biosensor Imaging of Redundant Deviation in Shifts (BIRDS) is a non-invasive MRI modality that maps absolute extracellular pH (pHe) by directly detecting paramagnetically shifted non-exchangeable protons of lanthanide-based exogeneous agents2, 3. Previously it has been used to characterize various tumors in the rat brain4-6 and to monitor the response to loco-regional treatment of liver cancer7. This study utilizes pHe mapping with BIRDS to differentiate ablated and non-ablated tumors in the setting of systemic immunotherapy of murine CRC.Methods:
Sixteen CRC were implanted in 8 BALB/c mice (Charles River Laboratories, Wilmington, MA, USA) with bilateral flank injections of 0.5 x 106 CT26 WT CRC cells (CRL- 2638; ATCC, Manassas, VA), and divided into 4 treatment groups randomly: Group 1 (SHAM) received injections of sham antibodies (InVivoMAb IgG controls), Group 2 (DICB - Dual Immune Checkpoint Blockade) was treated with anti-PD-1 and anti-CTLA-4 antibodies (Clone J43 & Clone 9D9; BioXcell, West Lebanon, NH, USA), III), Group 3 (tCRYODICB) with local Cryoablation in target tumors in left flank with systemic DICB and Group 4 (dCRYODICB) with systemic DICB without local Cryoablation in the distant off-target tumor in the right flank. pHe mapping with BIRDS was performed between 3 and 10 days after the initiation of treatment. A dose of 0.5mmol/kg TmDOTP5- was slowly injected at a rate of 60µl/h for 2 hours. The MR data was obtained on a 9.4T Bruker scanner (Billerica, MA). The T2 weighted images were obtained using a FOV of 28x28mm2, 128x128 matrix, 12 slices of 1mm thickness, TR=4s and TE=20ms. The BIRDS data was acquired using a 3D chemical shift imaging (CSI) sequence. Because paramagnetic probes like TmDOTP5- possess extremely short T1 and T2 relaxation times (0.1-10ms) and wide bandwidths (±200ppm), an ultrafast CSI sequence with short TR was used. Excitation was achieved using a dual-band 200µs Shinnar-Le Roux (SLR) RF pulse which selectively excited the H2, H3 and H6 peaks on either side of water. The CSI was acquired with a FOV of 23x15x17mm3, 659 spherical encoding steps, TR=5ms, 20min acquisition, and reconstructed to 23x15x17 with a voxel resolution of 1x1x1mm3. The pHe was calculated from the H2, H3 and H6 chemical shifts of TmDOTP5-.2, 3Results:
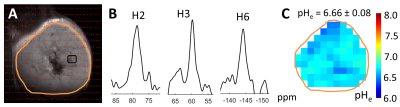
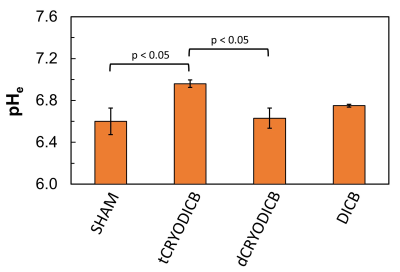
An example of pHe mapping with BIRDS of a murine CRC model is shown in Fig.1. The CSI signals (red) were overlaid onto a T2 weighted image (Fig.1A). The chemical shifts of H2, H3 and H6 protons of TmDOTP5- (Fig.1B) were used to calculate the pHe maps (Fig.1C). The results are summarized in Fig.2. The average pHe of untreated tumors (SHAM; pHe = 6.60 ± 0.13; n=5) was significantly lower (p < 0.05) than the average pHe of tumors treated with immuno-thermal ablation (tCRYODICB; pHe = 6.96 ± 0.04; n=3). Among the mice in the ablation groups, the target tumors demonstrated significantly lower acidity (p < 0.05) than the distant, off-target tumors (dCRYODICB; pHe = 6.63 ± 0.10; n=4). The average pHe of tumors treated only with dual checkpoint inhibitors (DICB, pHe = 6.75 ± 0.01; n=2) was not significantly different than any other groups (p > 0.05).Discussion:
We used BIRDS to assess extracellular pH changes in the microenvironment of murine CRC after immunotherapy with Immuno-Thermal Ablation. Immuno-Thermal Ablation with DICB and Cryoablation resulted in a significant pHe increase toward normalization in target murine CRC microenvironment compared to SHAM tumors (Fig.2) indicating a positive treatment outcome. A pHe increase was observed also in tumors treated only with DICB compared to SHAM tumors (Fig.2). In this experiment, cryoablation and DICB did not affect the microenvironment of distant off-target tumors, for which pHe remained similar to that of DICB treated only (Fig.2).Conclusion:
This work demonstrates the feasibility of mapping pHe with BIRDS in a murine CRC model after immunotherapy. pHe mapping could serve as a non-invasive imaging biomarker for tumor microenvironment assessment and monitoring of metabolic changes after immunotherapy with immuno-thermal ablation.Acknowledgements
This work was supported by a grant from the Department of Defense (W81XWH-17-1-0505).References
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a cancer journal for clinicians 2010;60: 277-300.
2. Coman D, Trubel HK, Rycyna RE, Hyder F. Brain temperature and pH measured by (1)H chemical shift imaging of a thulium agent. NMR in biomedicine 2009;22: 229-39.
3. Coman D, Trubel HK, Hyder F. Brain temperature by Biosensor Imaging of Redundant Deviation in Shifts (BIRDS): comparison between TmDOTP5- and TmDOTMA. NMR in biomedicine 2010;23: 277-85.
4. Coman D, Huang Y, Rao JU, De Feyter HM, Rothman DL, Juchem C, Hyder F. Imaging the intratumoral-peritumoral extracellular pH gradient of gliomas. NMR in biomedicine 2016;29: 309-19.
5. Huang Y, Coman D, Herman P, Rao JU, Maritim S, Hyder F. Towards longitudinal mapping of extracellular pH in gliomas. NMR in biomedicine 2016;29: 1364-72.
6. Rao JU, Coman D, Walsh JJ, Ali MM, Huang Y, Hyder F. Temozolomide arrests glioma growth and normalizes intratumoral extracellular pH. Scientific reports 2017;7: 7865.
7. Savic LJ, Schobert I, Peters D, Walsh JJ, Laage Gaupp FM, Hamm CA, Tritz N, Doemel LA, Lin M, Sinusas A, Schlachter T, Duncan JS, et al. Molecular imaging of extracellular tumor pH to reveal effects of loco-regional therapy on liver cancer microenvironment. Clinical cancer research : an official journal of the American Association for Cancer Research 2019 DOI: 10.1158/1078-0432.CCR-19-1702.
Figures