0286
Radial sampling and KWIC-reconstruction reduce motion artifacts and improve spatial resolution for DCE-MRI of mouse pancreatic cancer1Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Genetically engineered mouse (GEM) model of pancreatic ductal adenocarcinoma (PDA) recapitulates a dense stroma exhibited in human disease and is thus relevant for testing stroma-directed drugs. However, the lesion location makes it highly susceptible to motion. We present a multi-slice 2D saturation-recovery technique using golden-angle radial k-space sampling combined with KWIC reconstruction for DCE-MRI of PDA. This method minimizes respiration motion artefacts in PDA tumors and increases the effective temporal resolution of the AIF. Ktrans and kep maps derived from PK model fitting of DCE series exhibit adequate spatial resolution to reveal permeability and perfusion heterogeneity in the PDA tumor.
METHODS: A genetic engineered mouse (GEM) that carries Kras and p53 mutation in pancreas specific Cre allele, referred to as KPC model of PDA, was used in this study 2. MRI studies were performed on a 9.4T DirectDrive® system (Agilent) interfaced with a 12-cm gradient coil. ECG was monitored (SA Inc) and the core temperature was maintained at 37±0.2°C throughout MRI exams. Longitudinal relaxation time (T10) data were acquired using Look-Locker method we described previously 3, 4. DCE-MRI data were acquired using multi-slice 2D cardiac gated saturation recovery GRE employing golden-angle (111.246°) radial k-space sampling (TR/TE= 2x cardiac period ~ 200/0.91 msec, BW=100KHz, FOV=38 x 38 mm2). Multiple slice groups were planned with one cardiac short-axis slice and contiguous axial slices spanning the tumor. Radial DCE data were regridded to 256 x 256 matrix with or without employing k-space-weighted image contrast (KWIC) filter 5, 6 (Fig 1A). For KWIC, 25 views encoded the central k-space region, while 200 views encoded the outer-most regions. Non-KWIC reconstruction utilized 200 views (according to Nyquist criterion) throughout the entire k-space. Because k-space center is over-sampled in radial acquisitions, KWIC allows using fewer spokes at k-space center, thus effectively increasing temporal resolution with sliding window reconstruction 6. The AIF was extracted from the blood signal in heart left ventricle 3, 4, 7. After manual definition of tumor ROIs, DCE series, T1 map and individual-AIF were subject to pixel-wise least-squares fit to the “shutter-speed” pharmacokinetic model yielding parameter maps 3, 4.
RESULTS: The AIF was extracted from regridded images without and with KWIC reconstruction (Fig 1 B-C and D-E). The KWIC reconstruction provide sufficient temporal resolution to fully characterize the initial rise of the AIF. In contrast, the AIF produced from fully sampled images exhibits a blunted (smoothed) profile (Fig 1 F-G). KWIC reconstruction resulted in motion-free high resolution abdominal images of the PDA tumor (Fig 2A-C) despite the absence of respiration-gating. A comparison of selected images from the DCE series reconstructed with full sampling and KWIC demonstrated minimal differences in image quality between the two reconstruction methods (Fig 3A1-A5, 3C1-C5). The DCE signal time course for the two data sets over the tumor ROI are quite similar while the KWIC time course captures more time points during wash-in of the contrast agent (Fig 3B, D). Tumor T1, Ktrans and kep values (T1 = 2.5 ± 0.15 s, Ktrans =0.09-0.33 min-1, and kep = 0.5-1.6 min-1) obtained from parameter maps (Fig 3E, F, G) are similar to previously reported values 4 generated using Cartesian sampling methods at significantly lower spatial resolution.
DISCUSSION: For tumors in motion-susceptible locations such as pancreatic, liver, lung and breast cancer, motion-robustness is crucial for quantitative mapping of DCE-MRI parameters. By leveraging the intrinsic motion resistant properties of radial sampling with view-sharing (sliding window) features of KWIC filtering, we have obtained DCE images of murine pancreatic cancer free of respiratory motion artifacts simultaneously with observation of the AIF with high temporal resolution. The resulting DCE parameter values are consistent with what we have achieved in human DCE-MRI studies on clinical scanners 8-10.
CONCLUSION: GEM models of human cancer are being used more frequently in preclinical imaging studies. Quantitative DCE-MRI observation of the orthotopic tumors provided by these models is often challenging due to respiratory motion artifacts. Here we have demonstrated that Golden angle radial sampling combined with view sharing techniques can yield artifact free images with high temporal and spatial resolution in pre-clinical DCE-MRI studies.
Acknowledgements
This study was partially supported by U24CA231858 (Penn Quantitative Imaging Resource for Pancreatic Cancer), R21CA198563 and R01CA211337.References
1. Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005;7(5):469-483.
2. Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003;4(6):437-450.
3. Zhou R, Pickup S, Yankeelov TE, Springer CS, Jr., Glickson JD. Simultaneous measurement of arterial input function and tumor pharmacokinetics in mice by dynamic contrast enhanced imaging: effects of transcytolemmal water exchange. Magn Reson Med 2004;52(2):248-257.
4. Cao J, Pickup S, Clendenin C, et al. Dynamic Contrast-enhanced MRI Detects Responses to Stroma-directed Therapy in Mouse Models of Pancreatic Ductal Adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research 2019;25(7):2314-2322.
5. Song HK, Dougherty L. k-space weighted image contrast (KWIC) for contrast manipulation in projection reconstruction MRI. Magn Reson Med 2000;44(6):825-832.
6. Song HK, Dougherty L. Dynamic MRI with projection reconstruction and KWIC processing for simultaneous high spatial and temporal resolution. Magn Reson Med 2004;52(4):815-824.
7. Pickup S, Zhou R, Glickson JD. MRI estimation of the arterial input function in mice. Academic Radiology 2003;10:963-968.
8. Ge X, Quirk JD, Engelbach JA, et al. Test-Retest Performance of a 1-Hour Multiparametric MR Image Acquisition Pipeline With Orthotopic Triple-Negative Breast Cancer Patient-Derived Tumor Xenografts. Tomography : a journal for imaging research 2019;5(3):320-331.
9. Song HK, Dougherty L, Schnall MD. Simultaneous acquisition of multiple resolution images for dynamic contrast enhanced imaging of the breast. Magn Reson Med 2001;46(3):503-509.
10. Lin W, Guo J, Rosen MA, Song HK. Respiratory motion-compensated radial dynamic contrast-enhanced (DCE)-MRI of chest and abdominal lesions. Magnetic resonance in medicine 2008;60(5):1135-1146.
11. Dougherty L, Isaac G, Rosen MA, et al. High frame-rate simultaneous bilateral breast DCE-MRI. Magn Reson Med 2007;57(1):220-225.
Figures
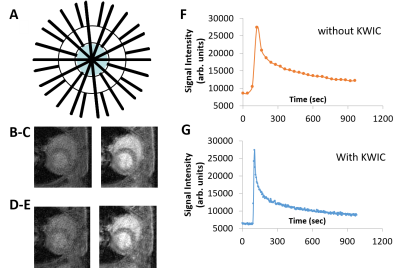
Figure 1 KWIC filter scheme and enhanced temporal resolution of AIF offered by radial k-space and reconstruction employed with KWIC filtering
(A) Scheme for KWIC reconstruction, where fewer views are used to fill the k-space center (blue) while 200 views are used at the edges of k-space. Radial DCE images obtained at 40 and 400 s respectively and extracted AIF from fully sampled images without KWIC (B-C and F) and with KWIC using 25/200 views center/outer per image (D-E, and G).
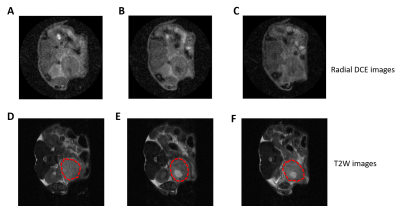
Figure 2 Motion-robustness and excellent spatial resolution afforded by radial DCE
DCE images at baseline (A-C) from three slices through the tumor (circle in red on T2W images in D-F) revealed lack of motion artefacts in DCE series which were acquired without respiration gating.
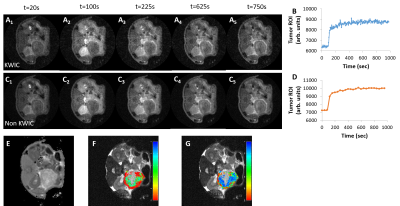
Figure 3 Radial DCE series, enhancement time course, T1 map, Ktrans and kep map of the tumor overlaid on T2W image.
Selected images from the DCE series reconstructed with full sampling (A1-A5) and KWIC (C1-C5) are compared. DCE signal time courses of the tumor ROI extracted from the two data sets are shown (B, D). Parameter maps of tumor T1 (E), Ktrans (F) and kep (G) are consistent with those obtained using lower resolution Cartesian sampling methods.