0284
Hippocampal MR Spectroscopy show chronic metabolic effects following cranial radiotherapy in childhood cancer survivors1Radiation Physics, Institute of Clinical Sciences, Sahlgrenska Academy, Gothenburg University, Göteborg, Sweden, 2Medical Physics and Biomedical Engineering, Sahlgrenska University Hospital, Göteborg, Sweden, 3Oncology, Institute of Clinical Sciences Sahlgrenska Academy Gothenburg University, Göteborg, Sweden, 4Health and Rehabilitation, Institute of neuroscience and physiology, Sahlgrenska Academy Gothenburg University, Göteborg, Sweden, 5Pediatrics, Institute of Clinical Sciences, Sahlgrenska Academy Gothenburg University, Göteborg, Sweden
Synopsis
Modern cranial radiotherapy (CRT) achieves high targeted doses of radiation to brain tumours, which has resulted in an increased population of long-term survivors of childhood cancer. Unfortunately, the CRT cause radiation damage to the healthy brain which results in cognitive deficits. In a group of adult childhood cancer survivors lower ratios of tNAA/tCho, Glu/tCho and Glu/tCr were obtained in the hippocampus for the patient group that received highest radiation doses to target volume as compared to the group that did not receive any CRT, indicating a still ongoing process of e.g inflammation, re-myelination and gliosis due to CRT
Introduction
Modern cranial radiotherapy (CRT) achieves high targeted doses of radiation to brain tumours, which has resulted in an increased population of long-term survivors of childhood cancer. Unfortunately, the CRT cause radiation exposure also to the healthy brain which results in cognitive deficits in the majority of the survivors1. Methods to predict, assess and ultimately prevent side effects due to CRT are therefore required. Late effects have been studied histologically, showing vascular abnormality, demyelination and white matter necrosis2, however, the pathogenesis of these damages are still poorly understood.Magnetic resonance spectroscopy (MRS) is a method that has the potential to provide an objective biomarker to study late effects of CRT. Several different metabolites in the brain can be measured, e.g. tNAA (a marker of neuronal density and function), creatine (tCr, part of the energy metabolism), choline (tCho, associated with cell membrane biosynthesis and metabolic turnover), glutamate (Glu, a major excitatory neurotransmitter) and myo-Inosytol (mIns, a marker of neuroglial cells and predominantly found in astrocytes).
The number of patient studies of late effects due to CRT is small and together with the believe that hippocampus is particularly sensitive to radiation injury our aim was to non-invasively study very late effects of CRT in the hippocampus using MR spectroscopy with short echo-time.
Methods
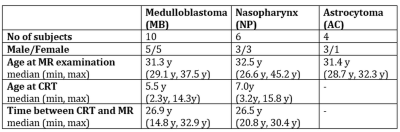
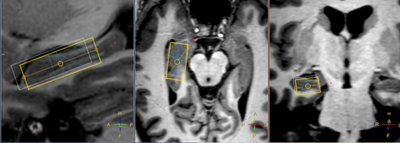
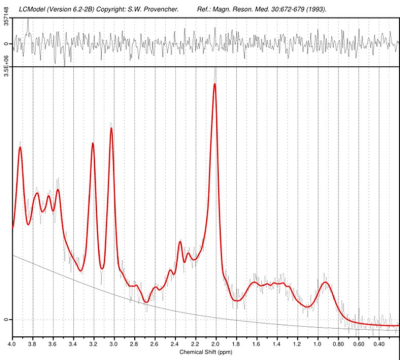
Adult childhood cancer survivors treated for three different types of tumours were studied: medulloblastoma (MB, n=10), tumours of the nasopharynx (NP, n=6) and low grade astrocytoma (AC, n=4). Characteristic data regarding the subjects and their treatment is summarized in table 1. Patients with medulloblastoma underwent surgery followed by chemotherapy and radiotherapy. The radiotherapy consisted of craniospinal irradiation, predominantly using two opposing lateral fields to the cranial volume. The median dose was 34.1 Gy delivered with fraction sizes of 1.5 to 2.0 Gy. A boost was then delivered sequentially to the posterior fossa and tumor bed, using either lateral opposing fields or 3D conformal treatment, to a cumulative median dose of 54.7 Gy. Of the six patients with tumours of the skull base or nasopharynx, two patients underwent surgery and five patients had chemotherapy. Radiotherapy to this group was delivered using 3D conformal radiotherapy. The median prescribed dose to the planning target volume was 48 Gy. Five of the six patients had hyperfractionated treatment with 1.6 or 1.7 Gy/fraction BID. Two patients with nasopharyngeal carcinoma had an additional brachytherapy boost of 6 Gy. All patients with low grade astrocytoma had surgical treatment alone and is in this study therefore used as a control group. The study was approved by the local ethical committee (ref no: 721-2015).All MRS measurements were performed on a Philips Gyroscan Achieva 3T release 5.1.7 to 5.4.1 (Philips Medical systems, Eindhoven, the Netherlands) with the 32 channel headcoil. MRI: 3D T1-weighted (T1W) turbo field echo for planning of MRS volumes in left and right anterior part of hippocampus (Fig. 1). MRS: PRESS TE = 40 ms, TR = 2000ms, CHESS water suppression, VOI size= 10*15*30 mm. Evaluation: LCModel version 6.2 with in house simulated basis sets using actual RF- and gradient objects. Only metabolites with Cramer-Rao lower bounds lower than 25% was used in the data evaluation: Glu, tCho, tCr, mIns,and tNAA. The mean concentrations for each metabolite of the left and right hippocampus was calculated as well as ratios to tCr and tCho, respectively. The statistical evaluation used Mann-Whitney U-test.
Results & Discussion
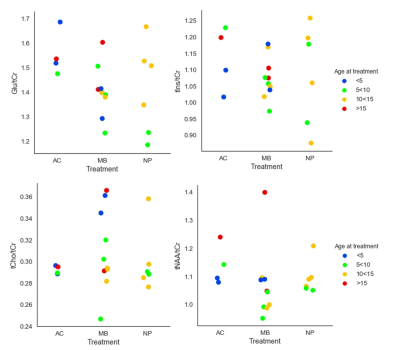
MR spectra from left and right hippocampus was obtained with high quality for all subjects (figure 2).Diagrams for ratios of tNAA, tCho, Glu and mIns versus tCr is shown in figure 3. The tNAA/tCr ratio had a non-significant trend towards lower values for the medullablastoma group as compared to the astrocytoma group, which agrees with previously published results3,4. Pospisil et al4 found a decrease of tCr in the hippocampus four months after radiation, which might also be an explanation for the moderate decrease of the NAA/tCr ratio in our study. The ratio Glu/tCr showed a statistically significant difference between the astrocytoma and medulloblastoma group (p=0.014), with lower values for the medullablastoma group.
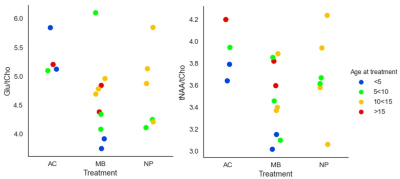
Metabolite ratios to tCho (figure 4) were also evaluated since this ratio could accentuate tissue damage due to irradiation. We found statistically significant differences between the astrocytoma and medulloblastoma group for tNAA/tCho (p=0.028) and Glu/tCho (p=0.014)). Increased Cho can indicate inflammation, de- or re-myelination and gliosis and lowered NAA can reflect neuronal damage5. Chen et al6 found in an animal study a decrease in the tNAA/tCho ratio after irradiation, which agrees with our results.
The age at treatment might be a confounding factor in these measurements and therefore is the data in diagram 3 and 4 colour coded for age at treatment using 5-year intervals.
No statistically significant differences were found between the medulloblastoma and nasopharynx groups.
Conclusion
In this group of adult childhood cancer survivors with very long follow-up time, lower ratios of tNAA/tCho, Glu/tCho and Glu/tCr were obtained in the patient group that received highest radiation doses to target volume as compared to the group that did not receive any CRT, indicating a still ongoing process of e.g. inflammation, re-myelination and gliosis due to CRT.Acknowledgements
The authors thank Philips Healthcare Clinical Science Group for support. The study was financed by grants from the Swedish Cancer Society, the King Gustav V Jubilee Clinic Cancer Research Foundation and the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreementReferences
1. Makale MT, McDonald CR, Hattangadi-Gluth JA et al. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol 2017; 13(1):52-64.
2. Lumniczky K, Szatmári T, Sáfrány G. Ionizing Radiation-Induced Immune and Inflammatory Reactions in the Brain. Front Immunol. 2017;8(517):1-13
3. Sundgren PC, Nagesh V, Elias A et al. Metabolic alterations: a biomarker for radiation-induced normal brain injury-an MR spectroscopy study. J Magn Reson Imaging. 2009; 29(2):291-7.
4. Pospisil P, Kazda T, Hynkova L et al. Post-WBRT cognitive impairment and hippocampal neuronal depletion measured by in vivo metabolic MR spectroscopy: Results of prospective investigational study. Radiother Oncol. 2017; 122(3):373-379
5. Balentova S, Hnilicova P, Kalenska D et al. Metabolic and histopathological changes in the brain and plasma of rats exposed to fractionated whole-brain irradiation. Brain Res. 2019; 1708:146-159
6. Chen H, Cheng YS, Zhou ZR et al. Long-term Brain Tissue Monitoring after Semi-brain Irradiation in Rats Using Proton Magnetic Resonance Spectroscopy: A Preliminary Study In vivo. Int J Radiation Research 2017;15(4): 363-369
Figures