0280
Patient Specific Planning of Thermal Magnetic Resonance: An EMF Simulation Study in Realistic Glioblastoma Multiforme Models1Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, 2Clinic for Radiation Oncology, Charité Universitätsmedizin, Berlin, Germany, 3MRI.TOOLS GmbH, Berlin, Germany, 4Experimental and Clinical Research Center (ECRC), joint cooperation between the Charité Medical Faculty and the Max-Delbrück-Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany
Synopsis
Ultrahigh-field MR employs higher radio frequencies (RF) than conventional MR and has unique potential to provide focal temperature manipulation and high resolution imaging in an integrated device (ThermalMR). The advantage of integrated therapy monitoring and guidance benefits thermal interventions in brain tumor treatments. To approach this goal this work examines the inter-patient variability in a heterogeneous group of glioma multiforme patients using EMF simulations. Our findings provide useful indicators as potential patient inclusion criteria for thermal treatment of brain tumors and form the technological basis for treatment planning and RF applicator developments en route to clinical applications of Thermal MR.
Introduction
Hyperthermia has proven beneficial as an adjunct treatment of Glioblastoma Multiforme (GBM)1. Advances in RF based thermal interventions have evoked developments in the design of annular2,3 or helmet shaped4 RF applicators for the treatment of brain tumors. Optimization algorithms used to confine the RF power deposition to the target volume (TV) are under constant revision5,6. The treatability of tumors strongly depends on a successful employment, monitoring and guidance of treatment planning algorithms as well as on suitable RF applicator design. Recognizing this challenge and opportunity, this EMF simulation study investigates the subject dependent thermal intervention plans (TIP) for six GBM patient-based realistic voxel models. For this purpose ten RF applicator configurations7 are examined using two phase and amplitude optimization algorithms8 for ThermalMR at 297 MHz.Methods
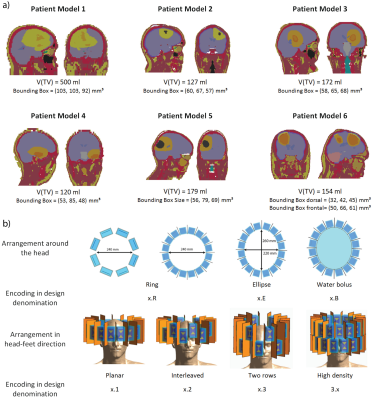
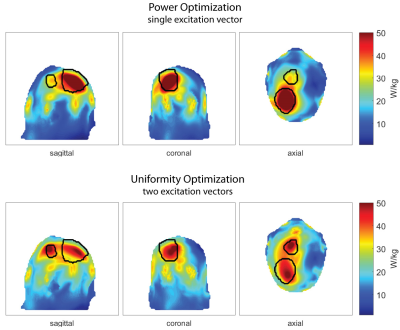
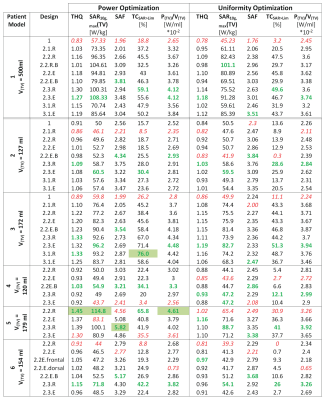
Six realistic patient voxel models were generated based on the delineation of the gross tumor volume (GTV), the clinical target volume (CTV), major anatomical structures and organs at risk9. Electromagnetic field simulations were performed for each combination of patient model and RF applicator design using Sim4Life10.From the channel-wise E- and H-field data, SAR10g11 matrices were calculated12. The two algorithms for phase and amplitude optimization aim at maximizing the RF power delivered to the target volume (TV), PTV 8. For algorithm 1, maximizing PTV is the sole optimization goal (Power Optimization) while algorithm 2 has the additional constraint to homogenize the RF power distribution over the TV (Uniformity Optimization).
The RF applicator configurations7 under investigation (Figure1) differ in a) the number of RF channels available for shaping the RF power deposition [8/16/32], b) the coverage of the head and thus focusing capabilities along the head-feet direction (z-direction) [planar/interleaved/two rows], c) the conformity to the human head [ring/elliptical] and d) the presence/absence of a water bolus for RF coupling from the antennae to the head.
The metrics chosen to analyze the TIP are
- SAR10g,max(TV): maximum SAR10g(TV)
- SAF: SAR amplification factor = ratio between SAR10g,mean(TV) and SAR10g,mean(healthy)
- TCSAR>Lim: target coverage with SAR10g≥40W/kg
- P(TV)/V(TV):
power delivered to the TV per ml target volume
- THQ: target-to-hotspot-quotient = ratio between SAR10g,mean(TV) and highest 1vol% SAR10g(healthy).
For all metrics, higher values indicate a better prospective treatment plan. The TIPs for each patient model were analyzed regarding the i) performance of the optimization algorithms, ii) number of RF channels and iii) brain coverage in z-direction. In order to draw conclusions about potential inclusion criteria for given brain tumor sites and sizes, all values are normalized to the inter-patient maximum of the single metric.
Results & Discussion
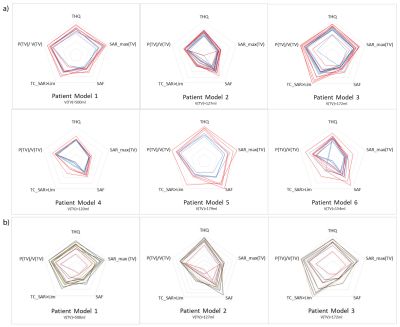
The patient-specific TIPs obtained for all RF applicator configurations for Power and Uniformity Optimization are summarized in Figure2a) with the absolute values of the metrics investigated being summarized in Table1. The Power Optimization (red) shows an overall better performance than the Uniformity Optimization (blue), often even for TCSAR>Lim. Further analysis will thus concentrate on the Power Optimization.The intra-patient dependency on the RF applicator design is addressed in Figure2b). It can be appreciated that coverage of the head and focusing capabilities in z-direction are not sufficient for the planar, single ring RF applicator designs (1.1.R and 2.1.R, red), leading to a suboptimal TIP. Moving from the 16-channel arrangements with large brain coverage (2.3.R/2.3.E) to the fully stacked 32-channel designs (3.1.R/3.1.E) did not enhance the TIP for any of the patient models. This finding benefits RF applicator developments because engineering constraints would be substantially offset by limiting the RF channel count to 16.
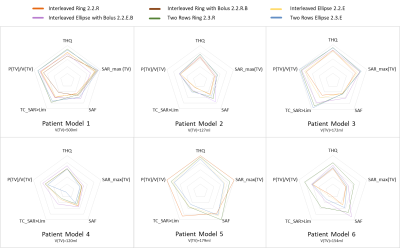
Figure3 shows the comparison of the 16 channel designs for all tumor models. The larger the TV, the more use can be made of the vertical extension of the RF applicators (green and blue lines for patients3,5&6). While the presence of a water bolus (orange) seems to be advantageous for the superficial TV in Patient5, it yields worst TIP for the deep seated tumor in Patient3.
A particular challenge was faced when computing the TIP for Patient6 who exhibits two separate TVs in close proximity. For this case, Uniformity Optimization yielded a solution of two excitation vectors, causing separate SAR10g maxima but poor coverage of the larger TV. Power Optimization resulted in a much better coverage of the larger TV but did not achieve any increased RF exposure in the smaller TV. Treating the TVs separately did not enable better TIPs.
Conclusion
Including a heterogeneous group of patients in the TIP and a range of RF applicator configurations into our EMF simulations has generated valuable insights. Our results demonstrate that i) the Power Optimization consistently outperforms the Uniformity Optimization, ii) planar, single ring arrangements of RF antennae do not provide sufficient control over the RF power delivery to treat high risk regions such as the brain and iii) the TIP enhancement effect of increasing the RF channel count plateaus, after which it does not outweigh the engineering effort.To conclude, our optimization algorithms and RF applicator designs favor target volumes where the TV’s extent along the head-feet direction approaches half of the wavelength (λ/2) in brain or tumor tissue. For the TIP of small or separate target volumes it is conceptually appealing to pursue the use of multiple and/or higher RF frequencies to enhance thermal intervention planning.
Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program under grant agreement No 743077 (ThermalMR).References
1. Sneed, P.K., et al., Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost±hyperthermia for glioblastoma multiforme. International Journal of Radiation Oncology* Biology* Physics, 1998. 40(2): p. 287-295.
2. Winter, L., et al., Design and Evaluation of a Hybrid Radiofrequency Applicator for Magnetic Resonance Imaging and RF Induced Hyperthermia: Electromagnetic Field Simulations up to 14.0 Tesla and Proof-of-Concept at 7.0 Tesla. PLoS One, 2013. 8(4): p. e61661.
3. Winter, L., et al., Thermal magnetic resonance: physics considerations and electromagnetic field simulations up to 23.5 Tesla (1GHz). Radiation Oncology, 2015. 10(1): p. 1.
4. Dobsicek Trefna, H., et al. Antenna Applicator for Microwave Hyperthermia Treatment of Pediatric Brain Cancer. in 8th European Conference on Antennas and Propagation, EuCAP 2014, The Hague, The Netherlands 6-11 April 2014. 2014.
5. Rijnen, Z., et al., Clinical integration of software tool VEDO for adaptive and quantitative application of phased array hyperthermia in the head and neck. International Journal of Hyperthermia, 2013. 29(3): p. 181-193.
6. Bellizzi, G.G., et al., Selecting the Optimal Subset of Antennas in Hyperthermia Treatment Planning. IEEE Journal of Electromagnetics, RF and Microwaves in Medicine and Biology, 2019: p. 1-1.
7. Oberacker E. et al., Radiofrequency applicator concepts for simultaneous MR imaging and hyperthermia treatment of glioblastoma multiforme: A 7.0 T (298 MHz) study, Proceedings of the 25th ISMRM Annual Meeting and Exhibition, Honolulu, Hawaii, USA
8. Oberacker E. et al., Power Considerations for Radiofrequency Applicator Concepts for Thermal Magnetic Resonance Interventions in the Brain at 297 MHz, Proceedings of the 27th ISMRM Annual Meeting and Exhibition, Montreal, Canada, 2019
9. Nadobny, J., et al. Fast and efficient generation of patient models for hyperthermia based on radiation therapy contours. in 32nd Annual Meeting of the European Society for Hyperthermic Oncology. 2018. Berlin: Strahlentherapie und Onkologie.
10. Sim4Life V3.4, ZurichMedTech, Zurich, Switzerland
11. IEEE, Recommended Practice for Determining the Spatial‐Peak Specific Absorption Rate (SAR) in the human body Due to Wireless Communications Devices: Measurement Techniques. 2003..
12. MatLab, The MathWorks Inc., Natick, Massachusetts, US
Figures