0275
Utilising preclinical 23Na MRI to probe the ionic microenvironment of breast cancer: developing novel diagnostics to probe treatment efficacy.
Andrew D James1,2, Theresa K Leslie1,2, Marie-Christine BD Labarthe3, Michaela Nelson1, Frank Riemer4, Gabrielle Baxter5, Joshua D Kaggie5, Fiona J Gilbert5, William Brackenbury1,2, and Aneurin J Kennerley2,3
1Biology, University of York, York, United Kingdom, 2York Biomedical Research Institute, University of York, York, United Kingdom, 3Chemistry, University of York, York, United Kingdom, 4MMIV, Haukeland University Hospital, Bergen, Norway, 5Department of Radiology, University of Cambridge, Cambridge, United Kingdom
1Biology, University of York, York, United Kingdom, 2York Biomedical Research Institute, University of York, York, United Kingdom, 3Chemistry, University of York, York, United Kingdom, 4MMIV, Haukeland University Hospital, Bergen, Norway, 5Department of Radiology, University of Cambridge, Cambridge, United Kingdom
Synopsis
Here we applied 23Na MRI, as part of a multiparametric imaging approach to measure ionic sodium concentration ([Na+]) levels in a longitudinal in-vivo mouse model of breast cancer. We investigated tumour [Na+] in response to neoadjuvant chemotherapy and ion channel inhibitors as a novel therapeutic means of reducing metastasis. Results show that [Na+] is decreased in tumour-bearing mice receiving standard chemotherapy. Data suggest that elevated tumour [Na+] in breast cancer may represent a potential imaging biomarker for malignancy and response to chemotherapy.
Introduction
In the present study we use preclinical 23Na MRI to measure ionic sodium concentration ([Na+]) levels in a longitudinal in-vivo mouse model to probe the efficacy of novel treatments targeting the tumour ionic microenviroment. Clinical intervention in early stage breast cancer is associated with a high survival rate, with late stage metastatic disease associated with poor prognosis. While recent progress has been made in understanding the role of the tumour microenvironment in breast cancer progression, little is known about how the ionic composition of tumours influences progression, metastasis and therapy response. Evidence indicates that tumour [Na+] is elevated in malignant breast tumours compared to surrounding tissue1,2, and this elevation is reduced following neoadjuvant chemotherapy3. Thus elevated tumour [Na+] represents an imaging biomarker for breast cancer lesions and therapy response, and the targeting of Na+ conductance routes through ion channels provides a novel therapeutic means of reducing metastasis.Methods
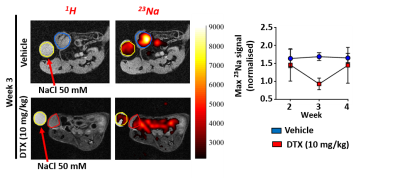
Tumour 23Na content was assessed in a xenograft longitudinal mouse model (female Rag2−/−/Il2rg −/−) of breast cancer (MDA-MB-231 cells). Following surgery (day 0), mice were split into 3 groups receiving: standard chemotherapy (docetaxel, 10 mg/kg i.p once weekly, n=7); a 23Na ion channel inhibitor (eslicarbazepine acetate, 200 mg/kg daily oral gavage, n=7); and vehicle control (n=14). 23Na MRI measurements were made at 7T in a pre-clinical MRI facility (Bruker BioSpec, 310mm bore) utilizing a quadrature 1H volume coil and a bespoke single channel 23Na surface coil. Subjects were scanned at the day 7, 14, and 21 time points. Mice were anaesthetised with 1-2% isoflurane mixed with 100% O2. Breathing rate was monitored throughout and body temperature maintained using a circulating warm water bath. At each time point we performed standard 1H high resolution (256*256, 40mm fov) T2, T2* structural and EPI-diffusion (single slice 96*64 matrix, 40mm fov, 3 gradient directions, 5 b-values 100-25000 s/mm2) imaging. 23Na imaging (figure 1a) used a single slice centric encoded FLASH based approach (32*32 matrix, 40mm fov, TR 50ms, TE 1.5ms, 400 average, and slice thickness 5mm). Data were compared to post-mortem electrophysiological slice recordings to better understand the underlying biological mechanisms.Results
Using 23Na MRI, we have established that tumour [Na+] is decreased in tumour-bearing mice receiving standard chemotherapy (docetaxel, 10 mg/kg i.p once weekly) after two cycles of treatment compared with untreated vehicle control (figure 1b). This standard chemotherapy inhibited tumour growth rate. Whether inhibited growth is a driver or a consequence to decreased tumour [Na+] compared with untreated tumours remains under investigation. Measurements of Na+ currents using the whole cell patch clamp method revealed no difference in Na+ conductance through voltage gated sodium channels between groups, suggesting that an alternative mechanism is responsible for the observed differences in tumour [Na+]. The antiepileptic medication eslicarbazepine acetate (a specific voltage gate sodium channel blocker, 200 mg/kg p.o. daily) had no effect on tumour growth, tumour [Na+], or tumour slice Na+ conductance.Conclusion & Discussion
We present data describing [Na+] measurement in cancer cells using 23Na MRI and complementary assessment of tumour cellularity via diffusion-weighted imaging and our preliminary findings assessing the effects of Na+ conductance route inhibitors on tumour [Na+] using the above approaches. Our results suggest that elevated tumour [Na+] in breast cancer may represent a potential imaging biomarker for malignancy and response to chemotherapy. Moreover, targeting of elevated tumour [Na+] should be investigated as a potential treatment avenue. Data will be used to help inform clinical translation of this technology.Acknowledgements
This project was funded by the CRUK (Project # A25922) and Breast Cancer Now (2015NovPhD572): PI Brackenbury.References
- Ouwerkerk R, Jacobs MA, Macura KJ, Wolff AC, Stearns V, Mezban SD, Khouri NF, Bluemke DA, Bottomley PA (2007) Elevated tissue sodium concentration in malignant breast lesions detected with non-invasive 23Na MRI. Breast Cancer Res Treat 106:151-160.
- Yang M, James AD, Suman R, Kasprowicz R, Nelson M, O’Toole PJ, Brackenbury WJ (2019) Voltage-dependent activation of Rac1 by Nav1.5 channels promotes cell migration. J Cell Physiol:1-23. 3.
- Jacobs MA, Ouwerkerk R, Wolff AC, Gabrielson E, Warzecha H, Jeter S, Bluemke DA, Wahl R, Stearns V (2011) Monitoring of neoadjuvant chemotherapy using multiparametric, (2)(3)Na sodium MR, and multimodality (PET/CT/MRI) imaging in locally advanced breast cancer. Breast Cancer Res Treat 128:119-126.