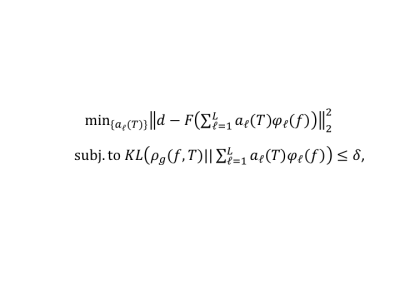0271
Dynamic Deuterium MRS Imaging of Brain Tumor with Enhanced Sensitivity and Spatiotemporal Resolution1CMRR, Department of Radiology, University of Minnesota, Minneapolis, MN, United States, 2Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 3Departments of Electrical and Computer Engineering, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 4Department of Neurosurgery, University of Minnesota, Minneapolis, MN, United States
Synopsis
Noninvasive MR-based metabolic imaging of brain tumor may offer new tools for clinic diagnosis and monitoring of tumor growth or assessment of treatment efficacy. One potential candidate is the dynamic deuterium MRS (DMRS) imaging technique recently developed. To reach its full potential, we integrated advanced data processing with D-MRSI to enhance its sensitivity or spatiotemporal resolution. We demonstrated in this pilot study that quantitative “Warburg Effect” map and kinetic time courses of deuterated metabolites can be achieved with good spatiotemporal scales in rat brain tumor using Deep-SPICE based deuterium MRSI, which could potentially be applied to brain tumor patients.
INTRODUCTION
Recently, we developed a novel metabolic imaging technique based on the in vivo deuterium (2H) MRS (DMRS) approach to quantitatively and simultaneously measure the cerebral metabolic rates of glucose consumption, TCA cycle and lactate (Lac) production.1 It has been shown that this neuroimaging technique is capable of assessing the “Warburg effect” in preclinical animal model and human patients with brain tumor;2-4 thus it could have a high potential for translational applications. However, similar to other X-nuclei MRS imaging techniques, 2H MRS imaging (DMRSI) also suffers from low detection sensitivity and low metabolite concentration that seriously limits its utility. In this work, we demonstrate that by applying the SPICE-based reconstruction method,5 the SNR of the DMRS signals can be significantly improved and 3D dynamic high-resolution deuterium images were obtained at 16.4T from rat brain tumor.METHODS
In vivo DMRSI measurement: Male and female Fischer rats with implanted gliosarcoma (GS-9L cells, Sigma-Aldirch) were scanned under 2% isoflurane anesthesia. Their femoral arteries and veins were catheterized for blood sampling, physiological monitoring and deuterated glucose infusion. All MR experiments were conducted on a 16.4 T/26 cm scanner (Varian/VNMRJ) using a passively decoupled 1H/2H surface coil. High-resolution dynamic 3D 2H-CSI with ~10 µL nominal voxel size (17x17x5 matrix and 28x28x24mm3 FOV) and 105s per CSI volume were acquired from the rat brains before, during and after the 2.5min i.v. infusion of D-Glucose-6,6-d2 (1.3 g/kg-BW dissolved in 2.5 mL saline, Sigma-Aldrich). All resonance signals of deuterated water, glucose (Glc), Glx (glutamate/glutamine) and Lac were analyzed and high-resolution images of Lac and Glx, and the Lac/Glx ratio images were generated.Deep-SPICE data processing method: Reconstruction of the desired spectral and temporal functions from the acquired 2H-MRSI data was accomplished by leveraging both physics model-based and data-driven priors. For the model-based priors, we used a subspace-based signal representation incorporating pre-learned spectral basis functions from high-SNR training datasets. For data-driven priors, we estimated the prior distributions of spectral and temporal functions using deep learning (DL) and incorporated them into the solution. Image reconstruction was formulated as solving the following optimization problem (voxel-by-voxel, ignoring the spatial coordinates):
Equation 1 (1)
where d is the measured data, F the imaging operator, φl(f) the pre-learned spectral basis, ρg(f,T) the ML-based prior, KL(·||·) the KL-divergence and δ some preset threshold.
RESULTS
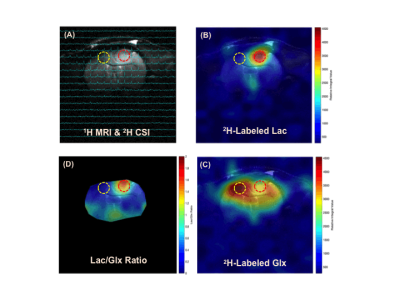
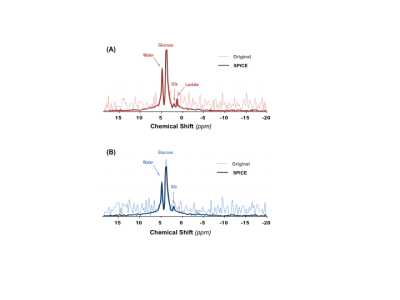
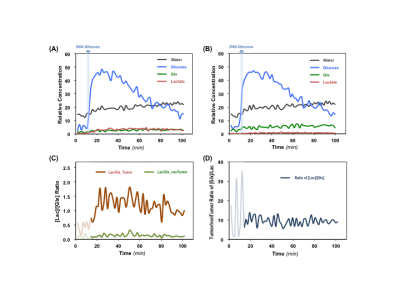
Figure 1 shows typical 2H MRS imaging obtained from a representative rat with brain tumor. The original natural abundance deuterium water signals distribution averaged from five CSI volumes pre-D66 infusion were displayed. The corresponding Lac and Glx images and Lac/Glx ratio maps generated using Deep-SPICE processing method were overlaid on the corresponding anatomic image where location of the brain tumor is evident. Figure 2 displays the original deuterium spectra obtained from a single 10µl voxel located either in tumor or non-tumor brain region as indicated in Fig. 1 with an acquisition time of Tacq < 2min. As a comparison, the corresponding spectra using Deep-SPICE processing method were shown in the same plot. Utilizing this newly developed post-data process method offering a large SNR improvement, we can evaluate crucial metabolic information and dynamics of the brain tumor from the time courses of key deuterium-labeled metabolites involved in the cerebral glucose metabolism. As shown in Figure 3, we found that the Lac/Glx ratio in the tumor region is ~10 times higher than the corresponding non-tumor region in this particular rat.DISCUSSION and CONCLUSION
The in vivo 2H-MRS imaging technique with improved sensitivity and spectral resolution at high/ultrahigh field has shown promises for studying abnormal glucose metabolism and TCA cycle activities in brain tumor.1-4 However, to further push the spatiotemporal resolution of DMRS-based metabolic imaging, it requires advanced data processing. The Deep-SPICE method was developed for improving the sensitivity and/or spatiotemporal resolution by effectively incorporating physics-based and data-based prior information. This approach was successfully applied in present study to evaluate the metabolic alterations in a rat model of gliosarcoma. We observed elevated lactate production and suppressed TCA cycle activity, a clear sign of “Warburg Effect” in the rat brain at the site of gliosarcoma,6 while normal glucose metabolism with high 2H-labeled Glx and low 2H-Lac were observed in corresponding normal appearing tissue. The improved DMRS sensitivity offered by the Deep-SPICE method allows us not only to generate high-resolution images of different metabolites or their ratios for assessing the brain tumor, but also to monitor their dynamic changes over the time, thus making it possible to calculate voxel-based metabolic rates from the dynamic data with largely enhanced temporal resolution and/or characteristics. Here we found that the differences between the tumor and non-tumor Lac/Glx ratios can reach ~10 times, which could be used as a sensitive measure of the tumor severity reflecting the degree of the “Warburg Effect” in brain tumor.This DMRSI-Deep-SPICE approach with further validation on metabolic quantification could provide a highly valuable and robust tool for DMRS-based metabolic imaging in brain tumor research and potentially for clinical translation.
Acknowledgements
NIH Grants: R01CA240953, R01MH111413, U01EB026978, P41 EB027061, P30 NS076408 and S10 RR025031.References
1. Lu, M., Zhu, X. H., Zhang, Y., Mateescu, G. & Chen, W. Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy. J Cereb Blood Flow Metab, 37:3518-3530 (2017).
2. Lu, M., Zhu, X. H., Zhang, Y., Low, W. & Chen, W. Simultaneous Assessment of Abnormal Glycolysis and Oxidative Metabolisms in Brain Tumor using In Vivo Deuterium 2H MRS Imaging in Proc. ISMRM p. 3962, (2016).
3. Lu, M., Zhu, X. H., Zhang, Y., Low, W. & Chen, W. High-resolution Deuterium MR Spectroscopic Imaging of the Warburg Effect in Brain Tumor. in Proc. Intl. ISMRM p. 4852, (2018).
4. De Feyter, H. M. et al. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci Adv 4, eaat7314, (2018).
5. Lam F, Liang ZP. A subspace approach to high‐resolution spectroscopic imaging. Magnetic Resonance in Medicine 71.4: 1349-1357, (2014).
6. Warburg O. On the origin of cancer cells. Science, 123(3191): 309-314 (1956).
Figures