0255
Noise and Bias Reduction in Two-Point Dixon Peripheral Nerve Imaging and Muscle Denervation Assessment1Radiology and Imaging, Hospital for Special Surgery, New York, NY, United States
Synopsis
High resolution, T2-weighted two-point Dixon is an effective technique for MR neurography (MRN) and can potentially also provide quantitative assessment of muscle ‘edema’ and fatty infiltration, which occur in acute and chronic muscle denervation, respectively. However, low SNR and residual off-resonance can introduce severe bias that impedes accurate interpretation. This study demonstrated that principal component analysis (PCA) denoising and off-resonance correction methods significantly improved proton density fat fraction (PDFF) measurements in 28 patient datasets and in signal simulations. Reduced bias allowed for further application of a proposed water-weighted processing enhances nerve conspicuity by suppressing perineural fat signal.
Introduction
MRN typically employs heavily T2-weighted (T2w), fat-suppressed sequences for visualizing peripheral nerve pathology and acute/subacute muscle denervation. The ‘water’ images derived from two-point Dixon method2 with T2w fast-spin-echo (FSE)1 provides an effective way for detecting T2 prolongation in nerves and edematous muscles; the derived ‘fat’ images allow for qualitative assessment of fatty infiltration, and quantitative proton density fat fraction (PDFF) measurement in chronically denervated muscle3,4. However, high in-plane resolution and long TE (~80ms) requirements for T2w MRN5 lowers SNR, severely biasing fat quantification and that inhibits attempts to increase spatial resolution. This bias is compounded by any residual, off-resonance remaining after B0 correction.Methods
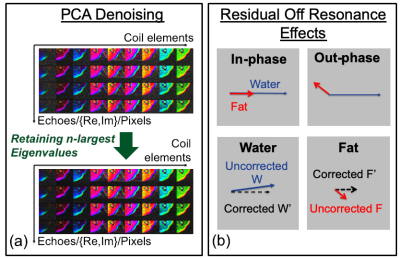
To reduce noise bias, principal component analysis (PCA) was applied6,7. The singular-value decomposition used in PCA was performed on the coil-element-by-(TE-pixel) matrix (Fig. 1a). Off-resonance correction (ORC) was applied by assuming that residual phase $$$\theta_f$$$ from the fat image (after accounting for B0), was due to fat components only. For small values of $$$\theta_F$$$ where $$$|\theta_F|<30^o$$$, water (W) and fat (F) signals obtained after complex reconstruction were corrected using $$$W'=W-\tt{tan}\it\theta_F \tt{sin}\it\theta_F |F|+i\tt{sin}\it\theta_F |F|$$$, and $$$F'=|F|/\tt{cos}\it\theta_F$$$ (Fig. 1b).Simulations of PDFF levels (0 to 1) and phase off-resonances (-90 to +90o) were performed, and at various noise levels (3 to 30%), with coil-sensitivities obtained from a 16-channel phased-array coil. For in vivo imaging, 9 subjects undergoing MRN were prospectively recruited for this IRB-approved study. Two-point-Dixon 2D T2w-FSE images were acquired as part of standard-of-care, with parameters varying depending on the body part imaged (TR/TE=3077-7520/78.7-88.5ms, FOV=10-36cm, matrix=320x224, 30-100 slices, slice thickness=1.2-4.0 mm, ETL=11-18, BW/pixel=244-391Hz). A total of 28 volumes (3 brachial plexus, 3 upper-arm/elbow, 5 forearm, 15 pelvis, 2 thigh) were acquired on 3T MRI (Signa Premier, and MR750, GE Healthcare) using either 16-channel flex or 32-channel torso coils. Raw data was reconstructed separately without and with PCA and ORC. A 3x3 kernel was used in PCA, with real and imaginary components separated to increase matrix size. Quantitative analysis was determined by measuring water image signal and PDFF from regions-of-interests (ROI) manually drawn on the water image – one nerve, subcutaneous fat, surrounding muscle (normal/abnormal, as determined qualitatively by absence/presence of diffuse signal hyperintensity and fatty infiltration of the muscle). An MSK radiologist with 6 years of MR experience qualitatively graded overall image quality, nerve conspicuity (1=poor, 2=average, 3=excellent), and pulsation/ringing artifacts (1=none, 2=mild, 3=severe). Generalized linear mixed models were used to compare differences in quantitative and qualitative measures from the PCA and ORC methods; 95% confidence intervals (CIs) and p-values for pairwise comparisons were adjusted using the Bonferroni method.
With improvements in signal bias, it was proposed to apply a water-weighted (WW) processing with $$$W'_{WW} =W^2 /(W+F)$$$ to further suppress fat signal for improving nerve conspicuity.
Results
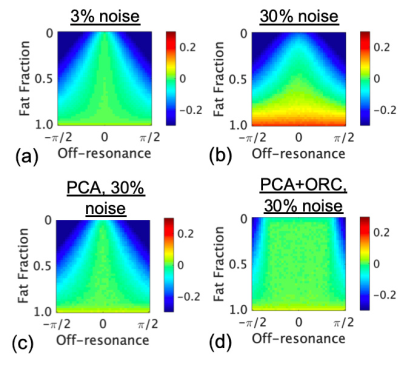
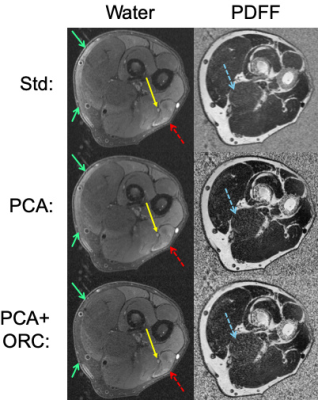
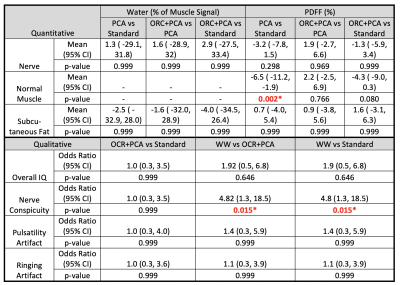
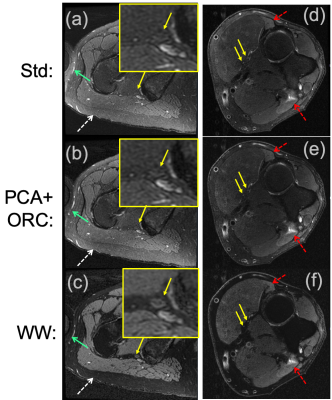
Simulations demonstrate that increasing noise-levels from 3% to 30% (Fig. 2a-b) resulted in positive bias of PDFF, whereas off-resonance resulted in negative bias of PDFF. PCA-denoising of the 30% noise-level data (Fig. 2c) reduced positive bias to levels similar to that from lower noise. Applying ORC further reduced bias from off-resonance (Fig. 2d), except when off-resonance was extreme (closer to +/-90 degrees).Fig. 3 shows water images and PDFF maps in one patient with a polyneuropathy, of unknown etiology, and denervated muscle. PCA conspicuously decreased PDFF in musculature, and reduced noise bias in pixels without signal (air and cortical bone). PCA, ORC and PCA+ORC increased relative water signal of nerves but not significantly; qualitatively, PCA+ORC did not alter overall image quality or nerve conspicuity. PCA and PCA+ORC reduced subcutaneous fat signal in the water image but not significantly (Table 1). However, PCA significantly decreased PDFF in normal muscle (-6.5%, p=0.002). ORC increased PDFF in normal muscle slightly, with the net PCA+ORC effect still leading to decreased PDFF (-4.3%, p=0.080).
Fig. 4a-b shows improved subcutaneous fat signal reduction from PCA+ORC in one subject. Fig. 4c shows conspicuous suppression of subcutaneous fat and fatty connective tissue from the WW processing, which significantly improved grading of nerve conspicuity (by +4.82 odds ratio, p=0.015), and to a lesser extent improved overall image quality (by +1.92 odds ratio, p=0.646).
Discussion
ROI measurements of water signal and PDFF agree with the predicted effects from simulations – i.e., that PCA and ORC reduce noise and off-resonance biases. Qualitative results suggest PCA and ORC did not alter perceived image quality or nerve conspicuity. Reduced bias in water/fat images allowed for WW processing, which significantly improved nerve conspicuity via improved suppression of perineural fat. While two-point-Dixon T2w-FSE is an effective pulse sequence for nerve visualization, reducing bias in Dixon processing could also provide improved quantitative measures for determining the extent of and staging of muscle denervation. In this cohort, only four subjects were diagnosed with nerve abnormalities; future work would correlate quantitative measures with clinical interpretations and electrophysiology results for the presence and severity of muscle denervation.Conclusion
PCA denoising and off-resonance correction techniques are promising to reduce bias in deriving quantitative water and fat measurements in two-point Dixon MR neurography and assessment of muscle denervation.Acknowledgements
This work was funded in part by research support from GE Healthcare. The authors thank Erin Argentieri, Jaemin Shin and Maggie Fung for assistance in analysis and pulse sequence support.References
1. Dixon W. Simple proton spectroscopic imaging. Radiology 1984;153:189–194 doi: 10.1148/radiology.153.1.6089263 .
2. Ma J, Son J, Bankson JA, Stafford JR, Choi H, Ragan D. A fast spin echo two-point Dixon technique and its combination with sensitivity encoding for efficient T2-weighted imaging. Magn Reson Imaging 2005;23:977–982 doi: 10.1016/j.mri.2005.10.005 .
3. Yu H, Shimakawa A, Hines CD, et al. Combination of complex‐based and magnitude‐based multiecho water‐fat separation for accurate quantification of fat‐fraction. Magnet Reson Med 2011;66:199–206 doi: 10.1002/mrm.22840 .
4. Karampinos DC, Holwein C, Buchmann S, et al. Proton Density Fat-Fraction of Rotator Cuff Muscles Is Associated With Isometric Strength 10 Years After Rotator Cuff Repair: A Quantitative Magnetic Resonance Imaging Study of the Shoulder. Am J Sports Medicine 2017;45:1990–1999 doi: 10.1177/0363546517703086 .
5. Filler AG, Maravilla KR, Tsuruda JS. MR neurography and muscle MR imaging for image diagnosis of disorders affecting the peripheral nerves and musculature. Neurol Clin 2004;22:643 682 doi: 10.1016/j.ncl.2004.03.005 .
6. Veraart J, Novikov DS, Christiaens D, Ades-aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage 2016;142:394 406 doi: 10.1016/j.neuroimage.2016.08.016 .
7. Lemberskiy G, Baete S, Veraart J, Shepherd T, Fieremans E, Novikov D. Achieving sub-mm clinical diffusion MRI resolution by removing noise during reconstruction using random matrix theory. Proc. ISMRM 2019:770.
Figures