0237
Feasibility of oscillating and pulsed gradient diffusion MRI to assess neonatal hypoxia-ischemia on clinical system1Children's Hospital, Zhejiang University School of Medicine, Hangzhou, China, 2Key Laboratory for Biomedical Engineering of Ministry of Education, College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, China, 3Department of Radiology, New York University School of Medicine, New York, NY, United States
Synopsis
Despite the success of diffusion time (td) dependent diffusion MRI in simulation and preclinical studies, clinical applications of the technique are challenged by difficulties in accessing short td, limited primarily by the clinical gradient system. This study demonstrated the feasibility of td-dependent dMRI using oscillating and pulsed gradients on a 3T clinical system to investigate neonatal hypoxic-ischemic encephalopathy (HIE). Results demonstrated that the td-dependency (ΔMD) increased in the deep gray matter of infants during the first year. In HIE patients, ΔMD increased in the basal ganglia of the severe and moderate HIE, as well as in the penumbra of lesions.
Introduction
Recent progress on diffusion-time (td) dependent diffusion MRI 1,2 opened new avenues to probe brain microstructure. The technique has shown to be particularly sensitive to certain cellular microstructural features, such as cell size and intra/extra-cellular space 3,4, which were useful in preclinical studies of tumor and ischemic injury 5,6. However, clinical applications of the technique remain difficult due to the challenges in achieving short td on clinical scanners with limited gradient strength. In this study, we investigated the feasibility of td-dependent MRI with pulsed and oscillating gradients in neonatal hypoxic-ischemic encephalopathy (HIE) on a clinical 3T system.Methods
Neonates with severe/moderate/mild HIE (n=7/6/5, respectively) and control subjects that were diagnosed as normal (term-born neonates (n=7), 1-year-old infants (n=7)) were enrolled in this study with IRB approval and parental consent. The basic information of the HIE neonates was listed in Figure 1.An oscillating gradient (OG)-dMRI sequence with trapezoid-cosine gradients was implemented on a 3T Phillips Achieva scanner (maximum gradient =80 mT/m) with the following parameters: an oscillating frequency of 33Hz (effective td=7.5ms), 2 oscillating cycles, b-value of 700 s/mm2, 4 diffusion directions, TE/TR = 168/10000 ms, FOV=180×180 mm, in-plane resolution = 1.4×1.4 mm, 10 slices with a slice thickness of 4ms, and 2 averages. Pulse gradient dMRI (PG-dMRI) was performed with σ/Δ = 60/82.8 ms, and the same parameters as OG-dMRI.
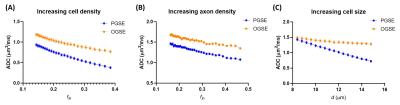
Monte-Carlo simulations using the Camino toolbox (http://camino.cs.ucl.ac.uk/) were performed to understand the relation between td-dependency and 1) brain development by simulating the effect of increasing cell density and axon density; and 2) HIE by simulating the effect of increasing cell sizes, using diffusion gradients identical to those used on the scanner. The simulation details were described in Figure 5.
Results
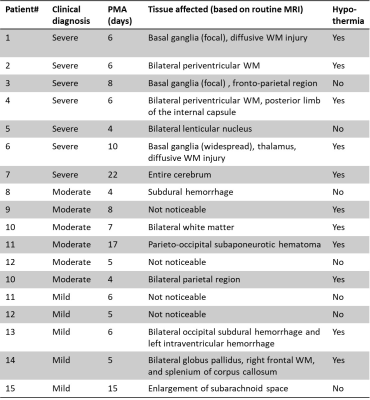
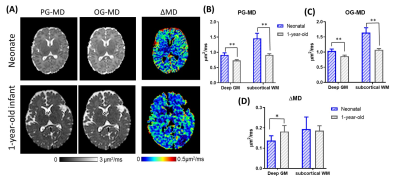
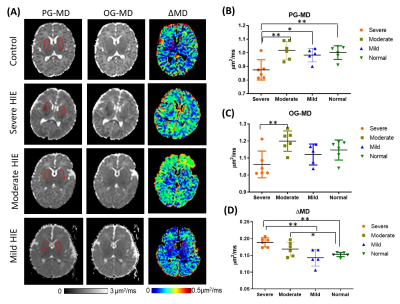
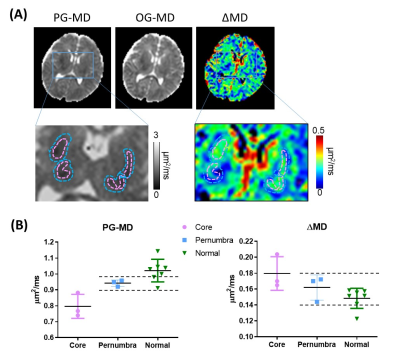
We first examined the td-dependency during brain development by comparing the mean diffusivities (MDs) in healthy neonates and 1-year-old infants. As expected, both OG-MD and PG-MD decreased with age. The td-dependency, evaluated by ΔMD (difference between OG-MD and PG-MD), significantly increased with age in the deep gray matter but not the WM (Figure 2). Cortical ΔMD was not reported due to the lack of resolution to accurately delineate the cortex in the neonates.Figure 3A demonstrated dMRI measurements in the basal ganglia (BG) in the control and HIE neonates that were diagnosed as severe, moderate, and mild injury according to clinical assessment. Focal or diffusive MD reductions in the BG and/or thalamus were visible in most of the severe HIE babies. Considerable elevation in ΔMD was observed in the BG (Figure 3B), and the effect size (23.7% elevation) was even larger than that of the PG-MD (12.6% reduction). In the moderate HIE neonates, a large variation was observed, and no difference in PG-MD was observed, compared with the control group, whereas the ΔMD showed a slight increase (p<0.05) than that in the control neonates. No difference was found between the mild HIE and control groups in any of the diffusion metrics. In three of the severe HIE neonates who had focal lesions in the BG or thalamus, we investigated dMRI signals in the core and penumbra of the lesions (Figure 4). As expected, PG-MD in the core was significantly lower than normal values but no apparent difference was found in the penumbra. In comparison, ΔMD in both the core and penumbra regions showed increased td-dependency. In other regions that we investigated (anterior/posterior subcortical WM and ventrolateral thalamus), no significant changes were observed, except for a marginal increase of ΔMD in the thalamus (p=0.03).
Simulations indicated that an increase in ΔMD can result from increasing cell density, but not it was directly associated with the axon density (Figure 5). This matched well with the experimental findings that ΔMD increased with age in the GM but the change was not significant in the WM. The simulation of cell swelling showed decreased PG-MD and increased ΔMD with increasing cell size, which also agreed with the experimental results. In addition, ΔMD appeared to more sensitive to cell swelling, e.g., 12% cell volume increase from a baseline of 10 µm resulted in a 9% increase in ΔMD and only a 3% decrease in PG-MD.
Discussion and Conclusion
Among the few clinical studies utilizing OG-dMRI, most of them focused on tumor microstructures 7,8. Baron et al. reported that, in stroke patients, short td actually reduced lesion contrasts in the MD maps, possibly due to axonal beading in the white matter 9. Our data are consistent with Baron et al. 9 as OG-dMRI, by itself, was less sensitive to edema than PG-dMRI. However, when looking into the td-dependency in the gray matter—the BG, which is known to be selectively injured in moderate-severe HIE babies who underwent an acute hypoxic-ischemic event 10,11, we found ΔMD was sensitive to the injury, not only in severe HIE but also in moderate HIE and penumbra of the lesion. The sensitivity of ΔMD was also supported previously preclinical studies of neonatal HIE 6,12. In addition, we found technical challenges of OG-dMRI (low b-values and long TE) were partially ameliorated in neonatal MRI, due to higher tissue diffusivity and longer T2 relaxation time in the neonatal brain than adult brains, making it feasible for neonatal MRI.Acknowledgements
This work was supported by the Natural Science Foundation of China (61801424, 81971606, and 91859201) and the Ministry of Science and Technology of the People’s Republic of China (2018YFE0114600).References
1. Stepisnik J. Time-Dependent Self-Diffusion by Nmr Spin-Echo. Physica B 1993;183(4):343-350.
2. Gore JC, Xu JZ, Colvin DC, Yankeelov TE, Parsons EC, Does MD. Characterization of tissue structure at varying length scales using temporal diffusion spectroscopy. NMR in biomedicine 2010;23(7):745-756.
3. Novikov DS, Jensen JH, Helpern JA, Fieremans E. Revealing mesoscopic structural universality with diffusion. Proceedings of the National Academy of Sciences of the United States of America 2014;111(14):5088-5093.
4. Xu JZ, Does MD, Gore JC. Dependence of temporal diffusion spectra on microstructural properties of biological tissues. Magn Reson Imaging 2011;29(3):380-390.
5. Reynaud O. Time-Dependent Diffusion MRI in Cancer: Tissue Modeling and Applications. Front Phys 2017;5.
6. Wu D, Martin LJ, Northington FJ, Zhang J. Oscillating-gradient diffusion magnetic resonance imaging detects acute subcellular structural changes in the mouse forebrain after neonatal hypoxia-ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2019;39(7):1336-1348.
7. Lima M, Kataoka M, Honda M, Ohno AK, Ota R, Ohashi A, Urushibata Y, Feiweier T, Toi M, Togashi K. Time-Varying Diffusion Patterns in Breast Cancer Linked to Prognostic Factors. 2019; Montreal, QC, Canada.
8. Xu J, Jiang X, Li H, Arlinghaus LR, McKinley ET, Devan SP, Hardy BM, Xie J, Kang H, Chakravarthy AB, Gore JC. Magnetic resonance imaging of mean cell size in human breast tumors. arXiv 2019;arXiv:1905.07818.
9. Baron CA, Kate M, Gioia L, Butcher K, Emery D, Budde M, Beaulieu C. Reduction of Diffusion-Weighted Imaging Contrast of Acute Ischemic Stroke at Short Diffusion Times. Stroke 2015;46(8):2136-2141.
10. Volpe JJ. Neonatal encephalopathy: An inadequate term for hypoxic-ischemic encephalopathy. Ann Neurol 2012;72(2):156-166.
11. Ghei SK, Zan E, Nathan JE, Choudhri A, Tekes A, Huisman TAGM, Izbudak I. MR Imaging of Hypoxic-Ischemic Injury in Term Neonates: Pearls and Pitfalls. Radiographics 2014;34(4):1047-1061.
12. Wu D, Martin LJ, Northington FJ, Zhang J. Oscillating gradient diffusion MRI reveals unique microstructural information in normal and hypoxia-ischemia injured mouse brains. Magn Reson Med 2014;72(5):1366-1374.
Figures