0236
Deep convolution neural network-based DWI tractography connectome analysis to predict language improvement after pediatric epilepsy surgery
Jeong-Won Jeong1,2,3,4, Min-Hee Lee1,2, Nolan O'Hara2,4, Eishi Asano1,3,4, and Csaba Juhasz1,2,3,4
1Pediatrics, Wayne State University, Detroit, MI, United States, 2Translational Imaging Lab, Children's Hospital of Michigan, Detroit, MI, United States, 3Neurology, Wayne State University, Detroit, MI, United States, 4Translational Neuroscience Program, Wayne State University, Detroit, MI, United States
1Pediatrics, Wayne State University, Detroit, MI, United States, 2Translational Imaging Lab, Children's Hospital of Michigan, Detroit, MI, United States, 3Neurology, Wayne State University, Detroit, MI, United States, 4Translational Neuroscience Program, Wayne State University, Detroit, MI, United States
Synopsis
Early surgery helps improve language function in pediatric epilepsy. We investigate if an advanced DWI approach combining deep convolution network-based tract classification with DWI connectome can help early surgery by providing preoperative imaging markers which indicate a high likelihood of postoperative language improvement. Our approach revealed two nodes in preoperative DWI data, including left middle temporal gyrus and left angular gyrus, of which preoperative local efficiency values are not significantly different in patients having postoperative improvement of receptive language, compared with age-matched healthy controls, which can be as effective imaging markers for prediction of the postoperative language improvement.
Introduction
Recent meta-analysis1 indicates that children with drug-resistant focal epilepsy (FE) should be referred for presurgical assessment in a timely manner. Early surgery can facilitate a more robust improvement of language function in subsets of FE children. This study investigates if an advanced imaging approach combining deep convolution neural network (DCNN)-based tract classification2-4 with diffusion-weighted imaging connectome (DWIC) can serve as accurate preoperative imaging markers capable of predicting postoperative language outcome.Methods
Pre- and postoperative DWI data of 25 FE patients (age: 10.21±2.5 years) were collected using a 3T MRI scanner with 55 isotropic gradient directions and b0 = 1000 s/mm3. For each patient, whole-brain tractography was reconstructed by tracking from 50 seeds in each white-matter-masked voxel and modeling diffusion peak orientations using MRtrix package (http://www.mrtrix.org/). AAL parcellation atlas (116 nodes, http://www.gin.cnrs.fr/en/tools/aal-aal2/) was used to create an adjacency matrix, S(u,v), in which each element defined a connection class, Ci, consisting of whole-brain total count of streamlines: fn, connecting both uth and vth nodes. To standardize a whole-brain backbone network5, B(u,v) from a given matrix, S(u,v), 1477 true positive classes of S(u,v) were identified by DCNN-DWIC tract classification2-4 (Fig. 1), where each tract of S(u,v): fn(x,y,z) was automatically classified into one of the 1477 true positive classes, Ci=1-1477, predefined for B(u,v). To construct a modular language network significantly associated with postoperative improvement of receptive language (Fig. 2A), functional correlation was performed between postoperative changes of 1) CELF6-based receptive language score: ΔNP = [scorepost – scorepre] and 2) tract count of B(u,v): ΔDWIC = [tract count in B(u,v)post – tract count in B(u,v)pre]. At each node of the modular network, Cohen’s d-value of preoperative local efficiency (LE)7 was evaluated using d-value = (m-μ)/s, indicating the distance between the LE value of the patient (m) and the mean of the LE values in the healthy control group (μ) in units of the standard deviation (s) of the LE values in the healthy control group. Finally, one-way ANCOVA (factor: ΔNP ≥ 0 vs. ΔNP < 0; dependent marker: Cohen’s d-value of preoperative LE, 5 covariates measured before surgery: age, seizure frequency, history of secondary generalized seizures, history of status epilepticus, total number of antiepileptic drugs tried) was performed to identify the nodes of which LE values were within or greater than the range of the control group, supporting a high likelihood of language improvement after surgery. The LE values of the identified nodes were used as new imaging markers for postoperative receptive language improvement.Results
Our modular network approach based on the DCNN-DWIC tract classification revealed two nodes in preoperative DWIC data, including the left middle temporal gyrus (L.MTG) and left angular gyrus (L.ANG), of which preoperative LE values are not significantly different in FE patients having ΔNP ≥ 0 (i.e., postoperative improvement/preservation of receptive language) compared with the age-matched healthy controls (Fig. 2B). These measures may represent potential markers predictive of postoperative improvement in receptive language function based on the preoperative DWIC data. That is, these preoperative d-values of LE significantly differed between ΔNP ≥ 0 and ΔNP < 0 (Fig. 3A), yielding a significant correlation between preoperative d-value and DNP (Fig. 3B). The subsequent binary regression analysis using d-values as predictors achieved high accuracy (92.9±4.5%) to predict postoperative improvement in random permutation test. This outstanding accuracy was achieved by our DCNN-DWIC tract classification significantly enhancing the group difference, ΔNP ≥ 0 vs. ΔNP < 0 in preoperative LE (rated by one-way ANOVA F-statistics) by 398% (F=2.05/8.14 without/with DCNN) and 259% (F=1.45/3.76 without/with DCNN) in two language hubs: L.MTG and L.ANG, respectively (Fig. 4).Discussion
Our findings provide new mechanistic insights as to whether the preservation (or increase) of local efficiency in a preoperative modular network predicts postoperative language improvement. Although preliminary, our data directly inform the clinical value of the proposed DCNN-DWIC analysis for accurate prediction of postoperative language improvement prior to resection, which may be expanded for prediction of other neurocognitive improvements such as full-scale IQ and fine motor skills.Conclusion
This study proposes a new DWIC approach using DCNN tract classification as an effective imaging tool for the prediction of postoperative language improvement. Our new imaging markers, at the level of specific modular networks, could be useful to identify the neural correlates and potential predictors of long-term, specific, neurocognitive consequences associated with surgical intervention.Acknowledgements
This work was supported by grants from the National Institutes of Health (R01 NS089659 to J.J. and R01 NS064033 to E.A.).References
[1] Pestana Knight EM, Schitz NK, Bakai PM, Korokian SM, Lhatoo SD, Kaiboriboon K. Increasing utilization of pediatric epilepsy surgery in the United States between 1997 and 2009. Epilepsia 2015;56:375-81. [2] Xu H, Dong M, Lee MH, O’Hara N, Asano E, Jeong JW. Objective detection of eloquent axonal pathways to minimize postoperative deficits in pediatric epilepsy surgery using diffusion tractography and convolutional neural networks. IEEE Trans Med Imaging 2019;38:1910-22. [3] Lee MH, O’Hara N, Jeong JW. Improving reproducibility connectome analysis using deep convolutional neural network model. Proc Intl Soc Mag Reson Med. 27: 3578, Montreal, Canada, May 11-16, 2019. [4] Jeong JW, Lee MH, Juhasz C, Asano E. Deep learning DWI connectome improves detection of language network alteration in epilepsy children. Annual Meeting of the Organization for Human Brain Mapping, 25: 2382, Rome, Italy, June 9-13, 2019. [5] Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, Beaulieu C. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex 2009;19:524-36. [6] Semel E, Wiig EH, Secord WA. Clinical evaluation of language fundamentals, fourth edition (CELF-4). Toronto, Canada: The Psychological Corporation/A Harcourt Assessment Company, 2003. [7] Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010;52:1059-69.Figures
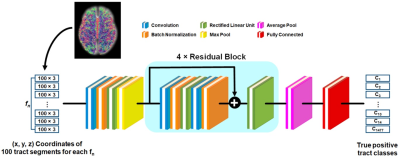
Figure
1. Schematic
architecture of the deep convolutional neural network (DCNN) to classify a
given fiber, fn into one of 1477 true positive tract classes, Ci=1-1477,
which are predefined for backbone network, B(u,v)4. Each colored square
represents a specific network layer.
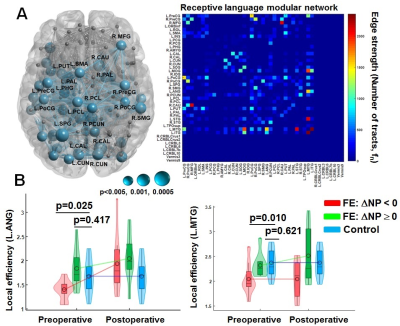
Figure
2. (A) Left: Nodes having significant correlation
between postoperative change in CELF receptive score (ΔNP) and imaging streamline count (ΔDWIC). Size of each sphere indicates p-value of Pearson’s correlation coefficient, R. Right: Receptive language modular network constructed
by nodes having a p-value < 0.005.
Color bar indicates the streamline count of each pair-wise connection. (B) Boxplot and histogram of local
efficiency obtained from pre- and postoperative DCNN-DWIC data.
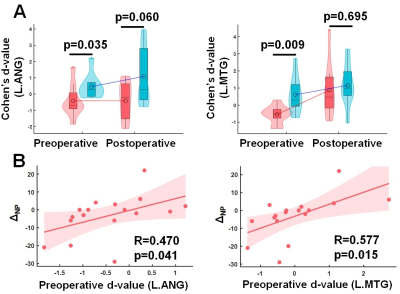
Figure
3. (A) Preoperative Cohen’s d-values of FE patients with ΔNP ≥ 0 (blue) significantly differ from those of patients with ΔNP < 0 (red) in two hubs: L.ANS (left) and L.MTG (right). (B) In each of the two hubs,
preoperative d-value was significantly correlated with ΔNP, suggesting that the preoperative d-value can be an
effective predictor for postoperative improvement.
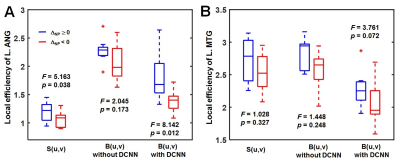
Figure
4. Statistical group
difference of local efficiency between FE patients with ΔNP ≥ 0 and FE
patients with ΔNP < 0, measured in (A)
L.ANG and (B) L.MTG. Three different connectome approaches using whole brain connectome network, S(u,v),
backbone networks, B(u,v) without DCNN and B(u,v) with DCNN were compared.