0235
Introduction of ultra-high field Magnetic Resonance Imaging in neonates: preparations and feasibility1Department of Radiology, University Medical Center Utrecht, Utrecht, Netherlands, 2Department of Neonatology, University Medical Center Utrecht, Utrecht, Netherlands, 3Department of Paediatric neurology, University Medical Center Utrecht, Utrecht, Netherlands, 4Department of Otorhinolaryngology and Head & Neck Surgery, University Medical Center Utrecht, Utrecht, Netherlands
Synopsis
The aim of this study was to investigate the safety and feasibility of 7T MRI in neonates. RF safety simulations showed that the global and peak specific absorption rates in a baby model do not exceed the specific absorption rates in adult models at 7T. Furthermore an (acoustic) noise damping hood was developed to guarantee hearing protection. In 10 neonates, we show that it is feasible to obtain good quality images at 7T; safety parameters (heart rate, peripheral oxygen saturation, peripheral temperature and comfort scales) were monitored before, during and after the MR scans, no (MRI related) adverse events occurred.
Introduction
Neonates with brain injury are at risk for neurodevelopmental delay. MRI is the gold standard to assess brain development and injury in neonates [1], and 3T scanners are now routinely used in neonates by many centers. However, predicting neurodevelopmental outcome often remains challenging. As ultra-high field MRI showed added value in adults in terms of resolution, SNR and CNR [2], 7T MRI could also be beneficial for clinical decision making in neonates.The aim of this study is to investigate the safety and feasibility of 7T MRI in neonates. We show the first MR images of neonates at 7T MRI and provide information about the study protocol and safety preparations.
Methods
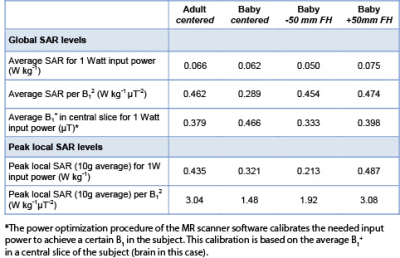
Safety preparationsPrior to the initiation of the in-vivo data collection, we evaluated the RF safety of the setup. Finite difference time domain simulations were performed (Sim4Life, Zurich Med Tech, Zurich) on a baby model (Charlie) of the virtual family [3], in different positions in the coil. We assumed full decoupling to the receiver coils. Peak local SAR (10g average) and global head SAR for 1 W input power were calculated.
Furthermore, we developed a prototype acoustic hood for noise protection that fits the 7T MR scanner, using a layer of 5 cm foam. A test setup (figure 1) with a dummy MR bore was made in a sound isolated booth to test the attenuation of acoustic noise with this prototype.
Feasibility
After these safety preparations we included clinically stable infants, between term (equivalent) age and the (corrected) age of three months. They underwent a 7T MRI immediately after their routine 3T MRI scan (both Philips Medical Systems, Best, Netherlands) using a 2-channel transmit-32 channel receive head coil (Nova Medical, Inc, Burlington, MA, USA). Noise protection was guaranteed by baby earmuffs and the acoustic hood. Safety parameters (i.e., heart rate, peripheral oxygen saturation, peripheral temperature and comfort scales) were monitored before, during and after the MR scans. The 7T MR protocol included T1w MPrage and T2w SSh TSE anatomical imaging, MR Venography (3D-PCA, 0.75x0.75x0.45mm, venc =7cm/s), Susceptibility Weighted Imaging (3D-FEepi, 0.55x0.55x0.27mm, epi factor 3, 3D flowcomp), MR Angiography (3D-Inflow, 0.3x0.3x0.2mm,) and single-voxel spectroscopy (MRS; sLASER, TE 36 ms, TR 5000 ms).
Results
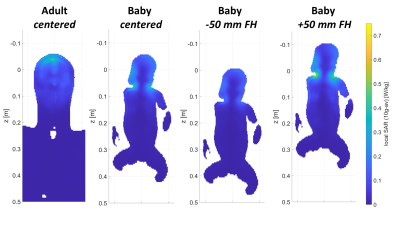
Safety preparationsRF safety simulations showed that the global SAR and peak local SAR of the baby model in centered position did not exceed the SAR of the adult model. However, when the baby model was positioned 50 mm from iso-center in head direction, global SAR levels and peak local SAR levels exceeded those of the adult model by +13% and +12%, respectively (Table 1 and figure 2). Therefore, positioning of the baby head in iso-center must be mechanically secured during scanning, for example by adding foam padding.
Acoustic noise protection was evaluated in a sound booth. The 7T hood attenuated the acoustic noise by 8.5dB. In comparison, the hood used at 3T [4] attenuated the noise by 7dB.
Feasibility
Ten neonates have been included. Temperature, heart rate and peripheral oxygen saturation were stable before, during and after MRI. No serious adverse events occurred.
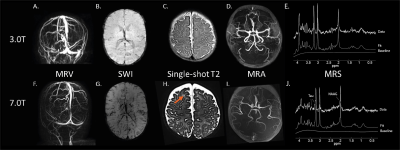
MRV at 7T shows improved visibility of the different veins and sinuses compared to 3T i.e. the superficial cerebral veins can be followed in more detail and the arterial circulation is better suppressed (Figure 3A+F).
On SWI at 7T, the deep venous circulation (i.e., the deep medullary veins) is better visible, (Figure 3B+G). Additional microbleeds have not been identified until now at 7T compared to 3T.
Single-shot T2-weighted imaging at 7T seems to be of improved quality. In one patient with a perinatal arterial stroke, perivascular spaces were found at 7T that were not visible at 3T (Figures 3C+H).
MRA is better at 7T than 3T, because less noise is visible at 7T making it easier to see the thickness and curves of the arteries (Figures 3D+I).
T1-weighted imaging does not yet meet the expected gain at 7T so it requires further improvement to incorporate the different T1 in neonates compared to adults.
MRS was of improved quality. For example, the patient shown in Figure 3E and 3J had an increased spectral resolution at 7T compared to 3T that better visualizes the different choline compounds and NAAG. It was possible to fit more metabolites with a Cramer-Rao lower-bound < 20% at 7T, such as NAAG and taurine.
Discussion and Conclusion
This pilot study shows both feasibility and safety of 7T MRI in ten neonates. A low SAR and acoustic noise could be demonstrated, while improved image quality at 7T compared to 3T was seen for SWI and single-shot T2-weighted imaging, caused by a shorter T2-relaxation time, improved spatial resolution and increased susceptibility. For MRS the increased chemical shift dispersion at 7T results in less overlap of different metabolite peaks.Further technical improvements, like optimizing B1+, incorporating T1 differences into flip angle and TR of the reported sequences are ongoing and additional sequences are being tested.
Neonatal imaging at 7T might enable physicians to assess the extent of injury on a microstructural level, e.g. diagnosing microbleeds or polymicrogyria, and thereby improve prediction of neurodevelopment.
Acknowledgements
EW and KA share first authorship. MB and JW share last authorship.References
1 de Vries L, Benders M, Groenendaal F. Imaging the premature brain: ultrasound or MRI? Neuroradiology. 2013;55:13–22.
2 Ladd ME, Bachert P, Meyerspeer M, Moser E, Nagel AM, Norris DG, Schmitter S, Speck O, Straub S, Zaiss M. Pros and cons of ultra-high-field MRI/MRS for human application. Prog Nucl Magn Reson Spectrosc. 2018;109:1-50
3. Christ A, Gosselin M, Christopoulou M, Gosselin M, Neufeld E, Moser H, et al. The Virtual Family — development of surface-based anatomical models of two adults and two children for dosimetric simulations The Virtual Family — development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys Med Biol. 2010;55:N23–38.
4. Nordell A, Lundh M, Horsch S, Hallberg B, Åden U, Nordell B, et al. The acoustic hood: a patient-independent device improving acoustic noise protection during neonatal magnetic resonance imaging. Acta Pediatr. 2009;98:1278–83.
Figures