0234
One-stage, language-dominant, opercular-insular epilepsy surgery with multimodal structural and functional neuroimaging evaluation1Neurosurgery, Royal Children's Hospital, Melbourne, Australia, 2Neuroscience Research group, Murdoch Children's Research Institution, Melbourne, Australia, 3Paediatrics, University of Melbourne, Melbourne, Australia, 4Developmental Imaging group, Murdoch Children's Research Institution, Melbourne, Australia, 5Neurology, Royal Children's Hospital, Melbourne, Australia, 6Neurology, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram, India, 7Medical Imaging, Royal Children's Hospital, Melbourne, Australia, 8Medicine and Radiology, University of Melbourne, Melbourne, Australia, 9The Florey Institute of Neuroscience and Mental Health, Melbourne, Australia, 10Speech pathology, Royal Children's Hospital, Melbourne, Australia, 11Clinical neuropsychology, Royal Children's Hospital, Melbourne, Australia, 12Melbourne School of Psychological Sciences, University of Melbourne, Melbourne, Australia
Synopsis
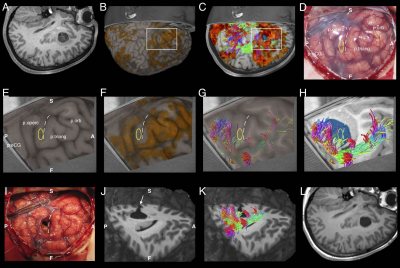
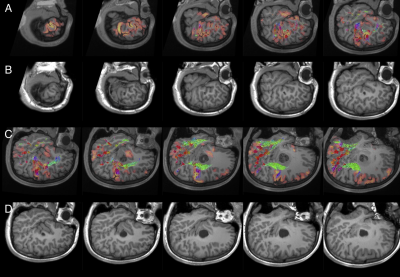
Language dominant, insular-opercular epilepsies are challenging to manage due complex seizure presentations and proximity to language cortex and associated white matter tracts. Most centres elected for extensive invasive stereo-electroencephalogram recordings and ablative procedures. We demonstrated in our retrospective cohort of 11 patients that focal resections can be undertaken safely and effectively using neuroimaging of seizures, lesions, language fMRI and high order tractography reconstructions based on a multi-fibre white matter modelling technique, and careful microsurgical techniques. This avoids risks associated with invasive procedures. Surgery performed under direct vision is more precise, likely safer, allows tailoring with intraoperative electrocorticography, and provides histopathology.
Introduction
Insular-opercular epilepsies are among the most challenging to manage due to their variable seizure semiology and complex seizure propagation patterns; the deep location of seizure foci with overlying and perforating arterial vessels; and their proximity to sensorimotor and language cortex and associated white matter tracts.1 These challenges lead to uncertainties and errors with electroclinical lateralisation and localisation of seizures; difficulties with detecting subtle lesions; the need to localise critical sensorimotor and language functions; and risk of neurological deficits, stroke and incomplete resection of epileptogenic lesion. Most centres avoid insular-opercular epilepsy surgery or undertake extensive invasive bilateral stereo-electroencephalogram (stereo-EEG) recordings2-6 and thermal ablative procedures.7,8 We report language-dominant, insular-opercular epilepsy surgery performed at our institution without invasive preoperative evaluation, utilising multimodal functional and structural neuroimaging in the preoperative planning and intraoperative execution.Methods
PatientsWe included 11 patients (age 8-18 years) who underwent localised epilepsy surgery in the language-dominant operculum and/or insula, between 2005-2019. Patients with large (perisylvian or multilobar) or superficial opercular (lateral to circular sulcus) lesions/resections were excluded.
MRI data was acquired on a 3Tesla MRI scanner with a 32-channel head coil. The main structural sequence was volumetric T1-weighted MPRAGE (0.8 mm3 isotropic voxels, TR/TE = 1900/2.69 ms).
DWI data
All DWI data processing and tractography reconstructions were performed using the MRtrix3 software. Multishell DWI data acquired with multi-band (MB) acceleration was conducted for 3 cases (b-values [no. of DW directions/ no. of b = 0 s/mm2] = 2800 s/mm2 [60/10]; 2000 s/mm2 [45/6]; 1000 s/mm2 [25/6]; MB factor = 3; 2.3 mm3 isotropic voxels; TR/TE = 3200/110 ms). Single-shell High Angular Resolution Diffusion Imaging acquired without MB acceleration for 8 cases (60 DW directions; b = 3000 s/mm2; 7 b = 0 s/mm2; 2.3 mm3 isotropic voxels; TR/TE = 7600/110 ms). A pair of b = 0 s/mm2 images with inverse phase encoding directions were obtained for susceptibility-induced geometric distortion correction.9
The DWI data was corrected sequentially for: thermal noise; Gibbs-ringing artefacts; eddy current and motion-induced distortions; EPI susceptibility-induced geometric distortions and b1-bias field induced inhomogeneity. White matter microstructural organisation was estimated based on multi-tissue Constrained Spherical Deconvolution modelling techniques.10,11
Arcuate fasciculus (AF) tractography
Tractography was performed using a probabilistic tracking algorithm with streamline reconstruction constrained by the Anatomically-Constrained Tractography framework.12 A target-specific tractography strategy based on region-of-interests placements in areas with known anatomical priors, guided by fibre-orientation distribution based directional encoded colour map. Arcuate fasciculus was reconstructed in three subcomponents: the long direct, anterior indirect and posterior indirect segments, based on previous cadaveric dissection13 and tractography studies.14
FMRI data and paradigms
Block design noun-verb generation and orthographic-lexical-retrieval language fMRI paradigms were used.15 The fMRI data was acquired using a gradient-echo EPI pulse sequence (1.9 mm in-plane voxel resolution; 3.0 mm slice thickness; TR/TE = 3000/40ms; 38 contiguous slices).
FMRI data was processed using FSL-FEAT. The processing steps included: non-brain removal, motion correction, B0 unwarping and registration to T1 using FLIRT, ICA-AROMA, spatial smoothing and high pass temporal filtering. Time-series statistical analysis was carried out using FILM with local autocorrelation correction. Z statistic images were thresholded using clusters determined by Z > 2.3 and a cluster significance threshold of P = 0.05, corrected for multiple comparison. The thresholded fMRI activate clusters were then multiplied by the combined grey matter (GM) and pathologic tissue masks to retain the GM component of the activation map for visualisation purpose.
Data analysis
Clinical, EEG, neuroimaging (language fMRI, PET, SPECT and AF tractography), operative and histopathology data were reviewed. Neuroimaging was scrutinised for lesion location, surgical corridor, resection completeness and proximity to language cortex/pathways. Postoperative seizure and language function outcomes were reviewed.
Results
All patients underwent microsurgical lesionectomy/corticectomy guided by localisation of the surgical target on MRI/PET/SPECT, cortically-limited language fMRI activation, AF tractography, rhythmic spiking on intraoperative electrocorticography (ECoG) and tissue texture. In one (latest) case, the combine tractography and fMRI activation maps were integrated into the neuronavigation device (Brainlab iPlan 3.0 Cranial) – which allowed for live, interactive imaging interrogations during surgery; guiding lesion resection by avoiding injury to eloquent language cortices and AF fibres. Cortical stimulation was performed in 5 awake patients, validating the fMRI and tractography findings. Histopathology was focal cortical dysplasia type II in 7, tumour in 3 and cavernoma in 1.Small strokes with transient aphasia occurred in 2 patients with posterior insular resections. Four patients required reoperations. Seizure outcomes (follow-up 1-6 years) were Engel class I (seizure free) in 10 patients and class II (rare seizures) in 1 patient. No patient had new postoperative permanent language deficits. Figures 1 and 2 showcase the imaging and corresponding intraoperative views from an exemplary patient.
Discussion and Conclusion
Focal resection in the language-dominant, operculum-insula can be undertaken in selected patients effectively and safely, by utilising multimodal neuroimaging of seizures, lesions, language fMRI and high-order AF tractography based on a multi-fibre white matter model, combined with intraoperative ECoG and careful microsurgical technique. We demonstrated comparable to favourable postoperative seizure, stroke and neurological profiles, compared with conventional approaches adopting extensive invasive stereo-EEG monitoring and ablation procedures.2-8 Surgery performed under direct vision is also more precise, likely safer, allows tailoring with intraoperative ECoG, and provides histopathology.Acknowledgements
This research was funded by the Royal Children’s Hospital Foundation (RCH1000 to SB and JYMY). This research was conducted within the Department of Neurosurgery and Neurology, Royal Children’s Hospital, and the Developmental Imaging and Neuroscience Research groups, Murdoch Children’s Research Institute at the Melbourne Children’s MRI Centre, Melbourne, Victoria. It was supported by the Royal Children’s Hospital Foundation, Murdoch Children’s Research Institute, The University of Melbourne Department of Paediatrics, and the Victorian Government’s Operational Infrastructure Support Program.References
1. Jobst BC, Gonzalez-Martinez J, Isnard J, et al. The Insula and Its Epilepsies. Epilepsy Curr 2019; 19(1): 11-21.
2. Dylgjeri S, Taussig D, Chipaux M, et al. Insular and insulo-opercular epilepsy in childhood: an SEEG study. Seizure 2014; 23(4): 300-8.
3. Proserpio P, Cossu M, Francione S, et al. Insular-opercular seizures manifesting with sleep-related paroxysmal motor behaviors: a stereo-EEG study. Epilepsia 2011; 52(10): 1781-91.
4. von Lehe M, Wellmer J, Urbach H, Schramm J, Elger CE, Clusmann H. Insular lesionectomy for refractory epilepsy: management and outcome. Brain 2009; 132(Pt 4): 1048-56.
5. Weil AG, Le NM, Jayakar P, et al. Medically resistant pediatric insular-opercular/perisylvian epilepsy. Part 2: outcome following resective surgery. J Neurosurg Pediatr 2016; 18(5): 523-35.
6. Gras-Combe G, Minotti L, Hoffmann D, Krainik A, Kahane P, Chabardes S. Surgery for Nontumoral Insular Epilepsy Explored by Stereoelectroencephalography. Neurosurgery 2016; 79(4): 578-88.
7. Hale AT, Sen S, Haider AS, et al. Open Resection versus Laser Interstitial Thermal Therapy for the Treatment of Pediatric Insular Epilepsy. Neurosurgery 2019; 85(4): E730-E6.
8. Perry MS, Donahue DJ, Malik SI, et al. Magnetic resonance imaging-guided laser interstitial thermal therapy as treatment for intractable insular epilepsy in children. J Neurosurg Pediatr 2017; 20(6): 575-82.
9. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage 2003; 20(2): 870-88.
10. Dhollander T, Raffelt D, Connelly A. Unsupervised 3-tissue response function estimation from single-shell or multi-shell diffusion MR data without a co-registered T1 image. ISMRM Workshop on Breaking the Barriers of Diffusion MRI 2016; Lisbon, Portugal; 2016.
11. Jeurissen B, Tournier JD, Dhollander T, Connelly A, Sijbers J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. NeuroImage 2014; 103: 411-26.
12. Smith RE, Tournier JD, Calamante F, Connelly A. Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. NeuroImage 2012; 62(3): 1924-38.
13. Martino J, De Witt Hamer PC, Berger MS, et al. Analysis of the subcomponents and cortical terminations of the perisylvian superior longitudinal fasciculus: a fiber dissection and DTI tractography study. Brain Struct Funct 2013; 218(1): 105-21.
14. Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol 2005; 57(1): 8-16.
15. Wood AG, Harvey AS, Wellard RM, et al. Language cortex activation in normal children. Neurology 2004; 63(6): 1035-44.
Figures