0233
MRI profiling of focal cortical dysplasia using advanced diffusion models1King's College London, LONDON, United Kingdom, 2UCL, London, United Kingdom, 3Great Ormond Street Hospital, London, United Kingdom
Synopsis
Lesion detection and sub-typing for focal cortical dysplasia (FCD), a frequent cause of drug-resistant epilepsy, remain challenging on conventional MRI. New diffusion models such as the spherical mean techniques (SMT) and the neurite orientation dispersion and density imaging (NODDI) provide measurements that are potentially more specific to abnormal tissue microstructure. Quantitative analysis of lesion profiling demonstrated significant changes on NODDI and SMT maps proportional to neurites density, as well on microscopic mean, radial and axial diffusivities. Moreover, signal changes specific to FCD lesions sub-types were observed on those maps, suggesting they can provide features useful for automated lesion detection.
Introduction
Focal cortical dysplasia (FCD) is a malformation of cortical development, and the most common cause of surgically treatable focal onset epilepsy in children1,2. Resective surgery is the most effective treatment to eliminate seizures, provided there is a well-characterised epileptic focus. Surgery is often performed on FCDIIa subtype, that contains dysmorphic neurons, and FCDIIb lesions that exhibit dysmorphic neurons and balloon cells1.FCD lesion detection and subtype identification remains challenging on conventional MRI. Diffusion MRI can probe tissue microstructure non-invasively, but current maps such as fractional anisotropy (FA) and mean diffusivity (MD) suffer from confounding signal effects related to fibre density/orientation dispersion which might decrease the specificity for lesion characterisation. Advanced diffusion models such as the spherical mean techniques (SMT)3,4 and neurite orientation dispersion and density imaging (NODDI)5, better account for those effects and potentially produce maps more specific to FCD lesions. This study aims to investigate lesion detection and subtype characterisation using the SMT and NODDI maps in paediatric cohort of FCD patients.
Methods
33 paediatric patients (10/4 years mean/s.d.) with radiologically defined FCD (histologically confirmed 18 FCDIIb ,4 FCDIIa), were scanned at 3T whole-body MRI system (Magnetom Prisma, Siemens Medical Systems, Germany), using a 20-channel receive head coil and body coil for transmission. T1-weighted, FLAIR and multi-shell diffusion-weighted (60 directions,b=1000,2200, acquisition time ~7 minutes) were acquired.Diffusion parameter maps were estimated using NODDI and SMT techniques applied to the diffusion-weighted data. Two expert neuro-radiologists manually delineated and scored the lesion visibility on the new diffusion parameters, FA, MD maps, and T1-weighted and FLAIR. Visual scores were compared between image groups.
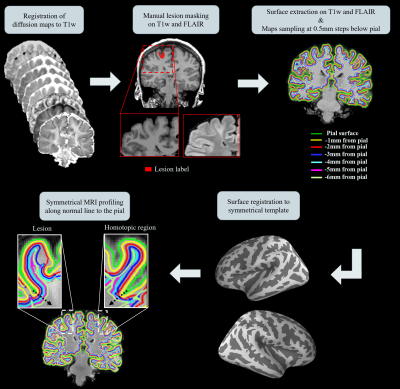
A surface-based approach was used to quantitatively investigate changes in diffusion parameters across different cortical, subcortical depths as described on Figure 1. This processing allowed analysing the signal profile changes within lesions and homologous regions. Profile changes in diffusion parameters were also statistically compared between FCD IIa and IIb.
Results
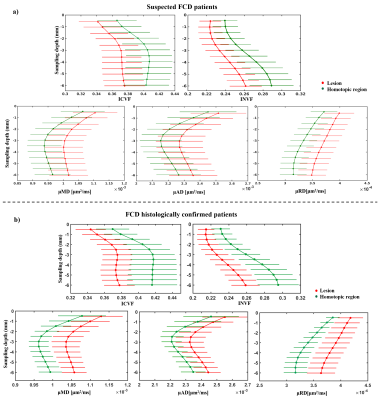
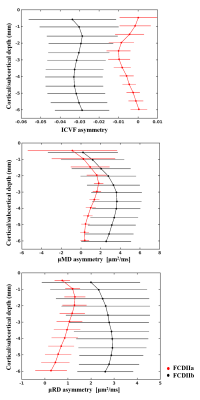
Figure 2 shows examples of FCDIIa and IIb lesions clearly visible on intracellular volume fraction (ICVF), intra-neurite volume fraction (INVF), microscopic mean, transverse and longitudinal diffusivity maps. Lesions were better or equally visualised on the ICVF maps in 13/33 individuals, and on INVF maps in 11/33 compared to FLAIR or T1-weighted images.Quantitative profiling demonstrated significant reduction of NODDI ICVF and multi-compartment SMT INVF, while SMT microscopic mean, transverse and longitudinal diffusivities were significantly increased in the lesions with respect to healthy homologous regions for the entire patients cohort and within the subset of patients with histologically confirmed FCD (see Fig.3a, b). FCDIIb exhibited greater changes than FCDIIa on the ICVF, microscopic mean and radial diffusivities (see Fig.4). No changes were detected on FA and MD maps.
Discussion
In agreement with the visual analysis quantitative investigation of ICVF, and additionally INVF microscopic mean, transverse and longitudinal diffusivity maps showed lesion-specific signal changes in suspected and histologically confirmed FCD. Both ICVF and INVF have been proposed as biomarkers of highly anisotropic structures, such as neuronal nuclei and glia. The within-lesion decreases are concordant with altered extracellular diffusion and increased extra-neurite volume measures performed on histology samples from surgical resections6. Those phenomena could also reflect the formation of additional diffusion barriers that may arise from loss of cortical stratification.The lack of lesion-specific changes on FA and MD might be explained by the fact that those maps are affected by healthy variability in underlying tissue properties including neuronal density, fibre orientation dispersion, axonal diameter and degree of myelination, which can hinder signal changes induced by pathological phenomena in our sample size.
Moreover, multi-compartment diffusion maps could help the characterisation of FCD subtypes as FCDIIb lesions exhibited enhanced signal changes on the ICVF, microscopic mean and radial diffusivities compared to FCDIIa, where signal alterations were subtle and affected layers closer to the pial surface.
Conclusions
The new diffusion maps showed changes in FCD lesions compatible with underlying disrupted tissue microstructure and could assist in the characterisation of the affected area and in the identification of the histological subtypes. Moreover, ICVF and INVF showed greater contrast than FLAIR in some cases and had consistent signal changes specific to FCD-type that suggest they can add value to current pre-surgical paediatric epilepsy imaging protocols.Acknowledgements
This research was funded by the Henry Smith Charity and Action Medical Research (GN2214). David Carmichael was supported by the King’s College London Wellcome/EPSRC Centre for Medical Engineering [WT 203148/Z/16/Z]. SA received funding from the Rosetrees Trust. TSJ receives funding from Great Ormond Street Children’s Charity, The Brain Tumour Charity, Children with Cancer UK, Cancer Research UK and the Olivia Hodson Cancer Fund.References
1. Blümcke I, Thom M, Aronica E, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia 2011;52:158–174 doi: 10.1111/j.1528-1167.2010.02777.x.
2. Harvey AS, Cross JH, Shinnar S, Mathern GW, Mathern BW, ILAE Pediatric Epilepsy Surgery Survey Taskforce. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia 2008;49:146–155 doi: 10.1111/j.1528-1167.2007.01421.x.
3. Kaden E, Kelm ND, Carson RP, Does MD, Alexander DC. Multi-compartment microscopic diffusion imaging. NeuroImage 2016;139:346–359 doi: 10.1016/j.neuroimage.2016.06.002.
4. Kaden E, Kruggel F, Alexander DC. Quantitative mapping of the per‐axon diffusion coefficients in brain white matter. Magn. Reson. Med. 2016;75:1752–1763 doi: 10.1002/mrm.25734.
5. Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 2012;61:1000–1016 doi: 10.1016/j.neuroimage.2012.03.072.
6. Vargova L, Homola A, Cicanic M, et al. The diffusion parameters of the extracellular space are altered in focal cortical dysplasias. Neurosci. Lett. 2011;499:19–23 doi: 10.1016/j.neulet.2011.05.023.
Figures