0232
Emergence of distinct structural reorganization patterns in children as a result of temporal lobe epilepsy surgery – beyond voxel-based analysis1Developmental Imaging and Biophysics Section, Institute of Child Health, University College London, London, United Kingdom, 2Forensic & Neurodevelopmental Sciences, King's College London, London, United Kingdom, 3Sackler Institute for Translational Neurodevelopment, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, United Kingdom
Synopsis
We compared Tract-based Spatial Statistics (TBSS) with a recent method which relies on non-statistical parametric mapping in a tractography derived anatomical framework – tract-based cluster analysis (TBCA) - to explore the effect of surgery in Temporal Lobe Epilepsy. In particular, we investigated differences in fractional anisotropy (FA) as an effect of surgery, and if those changes depended on operated hemisphere. We found the same patterns of increased FA on the corona radiata and decreased FA in tracts traversing the temporal lobe with TBSS, whilst TBCA allowed for an increase in anatomical specificity when interpreting differences in this group.
Introduction
The impact of brain surgery on the structural connectome in adults has been well explored, ranging from network connectivity analysis, comparison of functional activations before and after surgery, to traditional voxel-based analysis1,2,3. However, in a younger population, it becomes harder to tease apart these differences, which reflect brain plasticity and ability to reorganize following trauma, from the neurodevelopmental changes that naturally take place4. Furthermore, several studies use voxel-based techniques to assess changes and thus show regional changes without taking into account long range effects of local changes5. In this study, we investigated the effect of surgery on microstructural brain changes in children with Temporal Lobe Epilepsy (TLE) using traditional Tract Based Spatial Statistics (TBSS) and Tract Based Cluster Analysis (TBCA), a recently developed neuroimaging analysis method based on hypervoxels and diffusion MRI tractography.Methods
Data inclusion criteria: Subjects that undergone resective epilepsy surgery affecting the temporal lobes were selected for this study and divided into two groups, according to the side of the operation: 16 left TLE surgery subjects (median 11.50 years; inter-quartile range 8.5; 8 males) and 10 right TLE surgery (median 11 years; inter-quartile range 8.25; 3 males).Data acquisition: Each subject underwent a two shell DWI protocol on a Siemens Prisma 3.0T clinical system (Siemens Healthcare, Erlangen, Germany). Data were collected before and after surgery using a multi-band (acceleration factor of 2) diffusion weighted single-shot spin echo EPI; images were acquired for two sets of 60 non-collinear directions, using a weighting factor of 1000s.mm-2 and 2200s.mm-2 respectively, along with 13 additional T2-weighted (b=0) volumes. 66 axial slices of thickness 2.0mm were imaged, with 0.2mm slice gap, using a FOV=220×220mm final image resolution of 2.0×2.0×2.0mm; TE=60ms and TR=3050ms. In addition, a T1-weighted MPRAGE structural image was acquired using 176 contiguous sagittal slices, FOV=256×240mm and 1×1×1mm image resolution; TE=4.9ms and TR=11ms.
Data Processing and Statistical Analysis: DWI data were denoised using MRtrix’s6 implementation of the method developed by Veraart7. Furthermore, TOPUP and EDDY were used to correct for susceptibility distortions and to perform motion and eddy current correction8, before the DTI model was reconstructed and Fractional Anisotropy (FA) maps extracted for both pre and post-surgical data. Two processes were followed to investigate differences between pre and post-surgical FA in each group of subjects (Figure 1): 1) Standard TBSS9 pipeline, where the mean FA image was created and thinned to generate a mean FA skeleton with all projected data, followed by voxelwise repeated measures paired t-test using FSL Randomise and threshold free cluster enhancement; 2) A novel method for nonparametric cluster-level inference analysis - TBCA10 – which detects significant clusters formed by voxels anatomically connected and related to each other within a tractography template. Explanation variables (EVs) in the randomization tests included age at surgery, gender and days after surgery (all demeaned) and the effect of surgery was explored whilst regressing the other EVs. For both methods, resected areas were first removed to avoid bias in the final results.
Results and Discussion
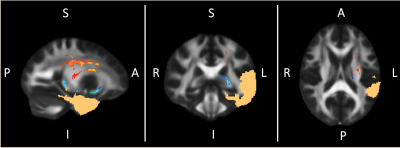
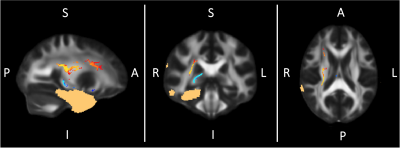
TBSS showed significant increases in FA in the ipsilateral corona radiata after surgery and significant FA decreases in the white matter surrounding the resected areas (Figure 2 and 3). Widespread effects on brain microstructure have been reported beyond the epileptic focus before surgery and the positive and negative FA changes we observe might simply respectively reflect readaptation to a “normal” state and Wallerian degeneration in the tracts connected to the resected area1,3. Given its ability to use the anatomical coherence between white-matter voxels and the connectivity information provided by tracts, TBCA detected multiple ipsilateral clusters of voxels belonging to distinct white matter tracts (Figure 4): significant increases in FA are highlighted by two clusters - corona radiata and cingulum - when the surgery is on the left, and one cluster when the surgery is on the right – corona radiate; furthermore, significant decreases in FA were associated with one cluster on the left – fornix/uncinated/inferior longitudinal fasciulus/fronto-thalamic connections – and two clusters on the right including the same regions. Although we found similar patterns of change with TBSS in both groups immediately after surgery, TBCA clusters differences with side of surgery might imply that, on the long-term, the brain will reorganize differently in each hemisphere4,9. However, a more in depth analysis reflecting changes overtime must be performed to confirm that those functional networks are affected differently as a result of the side of surgery. Finally, it is important to mention that FA changes may be confounded by orientational effects such as fibre crossings and orientation dispersion and more advanced methods should be employed to try to pick these apart10.Conclusion
We have applied a newly developed method to investigate the effect of TLE surgery in children, and compared it to traditional TBSS analysis. Immediately after surgery, emerging changes in FA are similar for both hemispheres, but we hypothesize that the long-term reorganization of the brain will be different depending on the side of the surgery. Finally, the increased anatomical specificity of TBCA may allow further characterization of those changes and inform interventions which can maximize the brain’s neuroplastic behavior and provide the best outcome following surgery.Acknowledgements
This research/study/project was funded by Fight for Sight and supported by the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London. GOSH BRC.References
[1] - Taylor PN, Sinha N, Wang Y, et al. The impact of epilepsy surgery on the structural connectome and its relation to outcome. NeuroImage: Clinical. 2018;18:202-214. doi:10.1016/j.nicl.2018.01.028
[2] - Liu TT, Nestor A, Vida MD, et al. Successful Reorganization of Category-Selective Visual Cortex following Occipito-temporal Lobectomy in Childhood. CellReports. 2018;24(5):1113–1127.e1. doi:10.1016/j.celrep.2018.06.099.
[3] - Yogarajah M, Focke NK, Bonelli SB, et al. The structural plasticity of white matter networks following anterior temporal lobe resection. Brain. 2010;133(8):2348-2364. doi:10.1093/brain/awq175.
[4] - Jeong J-W, Asano E, Juhász C, Behen ME, Chugani HT. Postoperative axonal changes in the contralateral hemisphere in children with medically refractory epilepsy: A longitudinal diffusion tensor imaging connectome analysis. Hum Brain Mapp. 2016;37(11):3946-3956. doi:10.1002/hbm.23287.
[5] - Otte, W. M., van Eijsden, P., Sander, J. W., Duncan, J. S., Dijkhuizen, R. M., & Braun, K. P. J. (2012). A meta-analysis of white matter changes in temporal lobe epilepsy as studied with diffusion tensor imaging. Epilepsia, 53(4), 659–667. https://doi.org/10.1111/j.1528-1167.2012.03426.x
[6] - J.-D. Tournier, F. Calamante, and A. Connelly, “MRtrix: Diffusion tractography in crossing fiber regions,” Int. J. Imaging Syst. Technol., vol. 22, no. 1, pp. 53–66, Feb. 2012.
[7] - J. Veraart, D. S. Novikov, D. Christiaens, B. Ades-Aron, J. Sijbers, and E. Fieremans, “Denoising of diffusion MRI using random matrix theory.,” NeuroImage, vol. 142, pp. 394–406, Nov. 2016.
[8] - J. L. R. Andersson and S. N. Sotiropoulos, “An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging.,” NeuroImage, vol. 125, pp. 1063–1078, Jan. 2016. [9] - Smith, S. M., Jenkinson, M., Johansen-Berg, H., Rueckert, D., Nichols, T. E., Mackay, C. E., … Behrens, T. E. J. (2006). Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage, 31(4), 1487–1505. https://doi.org/10.1016/j.neuroimage.2006.02.024
[10] – P. Luque Laguna, F.de Santiago Requejo, S.Williams , L. Goldstein3, M. Catani, F. Dell'Acqua, " Tract-Based Cluster Analysis", Proceedings of ISMRM annual Meeting Paris 2018, #0665. (Paris, France)
[11] - Tavakol, S., Royer, J., Lowe, A. J., Bonilha, L., Tracy, J. I., Jackson, G. D., … Bernhardt, B. C. (2019). Neuroimaging and connectomics of drug‐resistant epilepsy at multiple scales: From focal lesions to macroscale networks. Epilepsia, 60(4), 593–604. https://doi.org/10.1111/epi.14688
[12] - Huber, E., Donnelly, P. M., Rokem, A., & Yeatman, J. D. (2018). Rapid and widespread white matter plasticity during an intensive reading intervention. Nature Communications, 9(1), 2260. https://doi.org/10.1038/s41467-018-04627-5
[13] - Mollink, J., Kleinnijenhuis, M., Cappellen van Walsum, A.-M. van, Sotiropoulos, S. N., Cottaar, M., Mirfin, C., … Miller, K. L. (2017). Evaluating fibre orientation dispersion in white matter: Comparison of diffusion MRI, histology and polarized light imaging. NeuroImage, 157, 561–574. https://doi.org/10.1016/j.neuroimage.2017.06.001
Figures