0219
The neurocognitive effects of VSOP training in mild cognitive impairment (CogTE study): A phase-II clinical trial1Center for Advanced Imaging and Neurophysiology, University of Rochester, ROCHESTER, NY, United States
Synopsis
The current lack of effective pharmacological treatments for managing clinical symptoms in Alzheimer’s dementia highlights the urgent need for developing non-pharmacological interventions in the field. Here we report a phase II randomized controlled trial that examined the immediate and mid-term effect of a cognitive process based training on multiple cognitive domains in mild cognitive impairment. We found robust intervention effect on processing speed/attention and working memory. These cognitive improvements were associated with both activation changes and network changes involving ACC, a hub for maintaining successful cognitive aging. These results provide new insights about non-pharmacological interventions in preventing dementia.
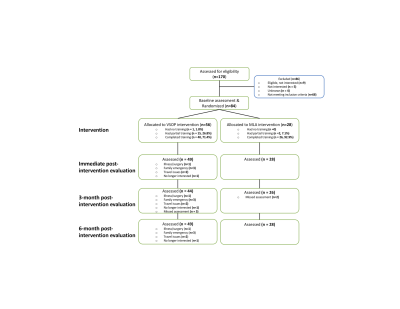
Methods: A phase II randomized, controlled, double-blinded clinical trial was conducted. Eighty-four adults with aMCI (mean age = 75 years), recruited from primary and secondary clinics, were randomly assigned to self-administered VSOP or MLA at a 2:1 ratio. VSOP training included five tasks involving processing speed and attention (PS/A). MLA included three mentally stimulating leisure activities. Both interventions lasted for six weeks, with four, one-hour sessions per week. Multiple cognitive, PS/A task-related fMRI, resting-state fMRI measures were assessed at baseline, post-intervention, and up to 6-month follow-up. CONSORT diagram is displayed in Figure 1.
Results: Intention-to-treat analyses was conducted. Task-fMRI analysis revealed one ROI, anterior cingulate cortex (ACC) where we found that, relative to MLA, the VSOP group had significantly greater increase in activation from baseline to post-test (Figure 2B). Notably, ACC is a region critical in supporting different cognitive function and differentiate different cognitive aging status. In contrast, an ROI in lateral occipital cortex (LOC) showed significantly increased activation from baseline to 6-month follow-up in MLA group relative to VSOP group (Figure 2B). For rsfMRI analysis, the ACC seed was related to four networks: central executive network (CEN), left, and right salience network (SN), and default mode network (DMN). Compared to the MLA group, the VSOP group had significant increases in FC strength within CEN and bilateral SN from baseline to post-test, or 6-month follow-up (Figure 2C). Data extracted from masks of ACC and LOC of task-fMRI and CEN and bilateral SN of rsfMRI were used in the subsequent analyses.
Across analyses, significant between-group differences that were higher in VSOP group included: UFOV from baseline to post-test (g = 0.51), working memory from baseline to post-test (g = 0.22) and 3-month follow-up (g = 0.30, Figure 2A), ACC activation (g = 0.50, Figure 2B), CEN (g = 0.75), left-SN (g = 0.29), and right-SN (g = 0.78, Figure 2C) from baseline to post-test, and left-SN (g = 0.56) from baseline to 6-month follow-up. Of note, the slight discrepancy between GEE vs. reliable improvement comparison results in working memory and left-SN may result from insufficient power for detecting any effect size < 0.40 (i.e., our proposed effect size). Significant between-group differences that were higher in MLA group included: episodic memory from baseline to 3-month follow-up and LOC activation from baseline to 6-month follow-up (Figure 2A & B).
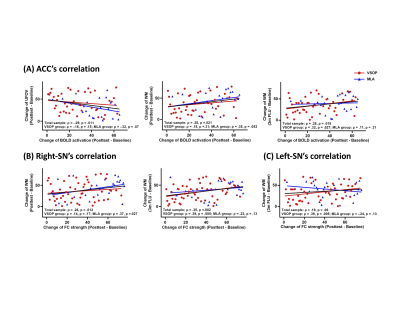
For the entire sample, increase of ACC activation was significantly related to improvement in UFOV and working memory from baseline to post-test, as well as improvement in working memory from baseline to 3-month follow-up (Figure 3A); enhancement of right-SN strength was significantly related to improvement in working memory from baseline to post-test and 3-month follow-up (Figure 3B). For VSOP group alone, enhancement of ACC activation and bilateral SN strength was significantly related to improvement in working memory from baseline to 3-month follow-up (Figure 3B and C). For MLA group alone, enhancement of right-SN was significantly related to improvement in working memory from baseline to post-test (Figure 3B). Of note, enhancement of LOC (i.e., found for MLA group in 6 months) was not related to any cognitive improvement.
Conclusion: VSOP training shows promise for causing improvements in PS/A and in working memory. Notably, these improvements are supported by relevant brain functional changes. Given our findings of pronounced changes shortly after training, it is possible that, however, VSOP may produce rapid, but relatively transient effects. Enhancing the adherence to VSOP training and adding booster sessions may strengthen and sustain the effects of training. Our results also raise new, yet unanswered, questions about VSOP training. Namely, it is unknown whether increasing the amount of VSOP training or adding booster sessions can enhance transferred effects in cognitive control or episodic memory; whether VSOP training would produce different effects in those with or without Alzheimer’s pathology.
Acknowledgements
The study was supported by NIH R01 NR015452 to Feng LinReferences
1. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280-292.
2. Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271-2281.
3. Wolinsky FD, Vander Weg MW, Howren MB, Jones MP, Dotson MM. A randomized controlled trial of cognitive training using a visual speed of processing intervention in middle aged and older adults. PLoS One. 2009;8(5):e61624.
4. Lin F, Chen DG, Vance D, Mapstone M. Trajectories of combined laboratory- and real world-based speed of processing in community-dwelling older adults. J Gerontol B Psychol Sci Soc Sci. 2013;68(3):364-373.
5. Ritchie SJ, Tucker-Drob EM, Deary IJ. A strong link between speed of visual discrimination and cognitive ageing. Curr Biol. 2014;24(15):R681-683.
Figures