0210
Effects of Levodopa Therapy on Cerebral Arteries and Brain Tissue Perfusion in Parkinson’s Disease Patients1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 2Neusoft Medical Systems Co., Ltd., Shanghai, China, 3Vascular Imaging Laboratory, Department of Radiology, University of Washington, Seattle, WA, United States, 4School of Computer Science and Technology, Beijing Institute of Technology, Beijing, China, 5Tsinghua University Yuquan Hospital, Beijing, China
Synopsis
Parkinson’s Disease (PD) has shown to be associated with cerebrovascular abnormalities, but its non-dopaminergic pathological mechanism is less studied. This study investigated the regulatory effect of levodopa, the most-commonly used therapy for PD, on cerebral arteries and blood flow. 57 PD patients and 17 age-matched healthy controls were scanned for artery morphologic and cerebral perfusion imaging at baseline, then the patients were re-scanned 50 minutes after taking levodopa. Results indicated that levodopa elevated blood perfusion level of PD brains to normal levels and dilated proximal arteries. Plus, blood perfusion showed related to motor syndrome scale post-levodopa.
Introduction
In Parkinson’s disease (PD) patients, cerebral hemodynamic changes and perfusion disturbances have been widely reported1. As the most effective therapy, levodopa’s biochemical mechanism has been well-studied, whereas its effect on cerebral arteries and blood flow is unclear. To fill the gap, pseudo-continuous arterial spin labeling (PCASL) technique for blood perfusion imaging, and simultaneous non-contrast angiography and intraplaque imaging (SNAP) for artery morphological imaging were employed. By applying the imaging techniques together, we aim to investigate the influence of levodopa on cerebral arteries and perfusion in PD patients quantitatively.Methods
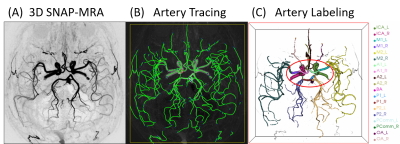
Fifty-seven PD patients (56.4 ± 9.8 years, 26 males) and 17 age-matched healthy controls (AMC) (56.5 ± 8.7 years, 9 males) were scanned on a 3T MRI scanner (Philips Healthcare, Best, The Netherlands) using a 32-channel SENSE head coil. Each PD patient was scanned twice before (“OFF” levodopa) and after (“ON” levodopa) levodopa intaking (300 mg) using the above protocol with a gap of 50 minutes. Before each scan, the Unified Parkinson’s Disease Rating Scale 3.0 (UPDRS-III) was recorded for each patient. The MRI scan protocol contains three sequences: 1) 3D turbo field echo (TFE) sequence as an anatomical scan, FOV = 256×160×256 mm3, resolution = 1 mm isotropic, TR/TE = 7.6/3.7 ms, scan time = 3min 46s. 2) 3D SNAP, FOV = 160×160×160 mm3, resolution = 0.8 mm isotropic, TR/TE = 10/5.6 ms, TI = 500 ms, flip angle = 11°, scan time = 5min 7s; 3) PCASL, FOV = 240×240×132 mm3, resolution = 3×3×6 mm3, TR/TE = 4013/12 ms, SENSE acceleration = 3, label duration = 1650 ms, post labeling delay (PLD) = 1575 ms, scan time = 4min 9s. SNAP-MRA images were generated by background suppression and minimum intensity projection from SNAP raw images using phase-sensitive reconstruction technique. Then they were analyzed using a semi-automated artery tracing and labeling tool (iCafe)2 for artery feature extraction (an example of labeled arteries is shown in Fig.1). The automatically generated artery traces were checked and corrected by two experienced specialists (20th year neurosurgeon and 12th year neuroradiologist both had trained to review images of iCafe with 45hrs). As outputs, mean radius and length of arteries were extracted. Cerebral Blood Flow (CBF) maps were calculated from PCASL images with in-house Matlab code, before which raw PCASL images were motion-corrected and spatial smoothed. To be noted, a gray-matter mask was applied to calculate the blood flow within gray matter regions (GM-CBF). We also calculated the regional CBF values corresponding to arteries that we measured in the SNAP images based on two artery territory templates3,4. All above imaging processing procedures were applied to all PD and AMC images. Post-hoc statistics of above imaging matrices includes: (1) Mann Whitney U tests on PD vs. AMC; (2) Wilcoxon matched-pairs signed rank tests comparisons between levodopa “OFF” vs. “ON” in the PD patients; (3) linear regressions on UPDRS-III scores to the imaging metrics in both levodopa “OFF” and “ON” state.Results
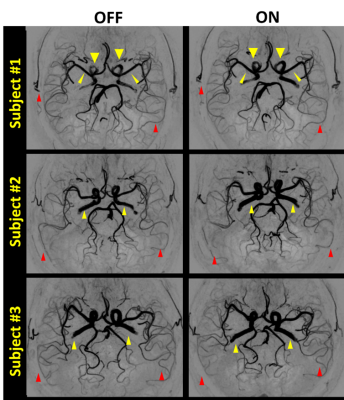
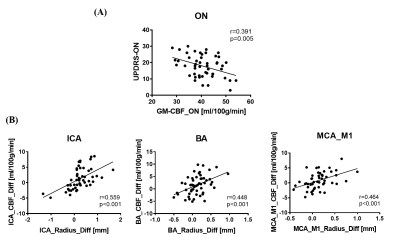
UPDRS-III scores decreased significantly after levodopa (p < 0.001), suggesting the motor symptoms were relieved. The PD patients showed lower GM-CBF in all arterial territories than AMC while increased to normal levels after taking levodopa. As for the levodopa “OFF” vs. “On” in the PD patients, the total artery length decreased after drug therapy, which indicated the reduced overall cerebral blood flow velocity as our SNAP technique is sensitive to blood flow speed5. We also observed increase of artery radius in proximal arteries. An example is shown in Fig. 2. Quite reasonably, changes in the artery radius are correlated to corresponding perfusion territories (as shown in Fig. 3B). Specifically, consistent changes of dilation and perfusion was found in the internal carotid arteries (ICA, p<0.001, r=0.559), basilar artery (BA, p<0.001, r=0.448), and the segment M1 of middle cerebral artery (MCA_M1) (p<0.001, r=0.464). Raised whole-brain GM-CBF is related to UPDRS-III (p=0.005, r=0.391, as shown in Fig. 3A). With the combined evidences from the increased blood flow, the lower flow velocity, and the dilated arteries, we believe that levodopa increased cerebral perfusion by dilating arteries.Discussion
This study for the first time validated the beneficial effect of levodopa in increasing global and regional CBF to normal levels through artery dilation. However, from a pathological view, how are these changes induced by levodopa and whether it can help to explain the drug resistance and side effects of levodopa are still uncertain. Interestingly, in our study, levodopa dilated proximal arteries significantly in a short time (50 minutes), which has never been reported before. Considering that the occlusion and stenosis are largely identified in subjects with ischemic stroke, we suggest the vasodilation effect of levodopa may be a potential solution for early ischemic stroke. We acknowledge and recommend the combination of different imaging modalities in PD studies. The small sample size of AMC is the main caveat of this study.Conclusion
Levodopa can increase the gray matter blood flow of PD patients by dilating the proximal arteries, and its ability to dilate the proximal arteries is related to its short-term therapeutic effect.Acknowledgements
This work was supported by the National Key Research and Development Program of China (grant numbers: 2017YFC0108900 and 2017YFA0205904) and National Natural Science Foundation of China (grant numbers: 61571258 and 61971258).References
1. Ma Y, Huang C, Dyke JP, Pan H, Alsop D, Feigin A et al. Parkinson's Disease Spatial Covariance Pattern: Noninvasive Quantification with Perfusion MRI. Journal of Cerebral Blood Flow & Metabolism 2010; 30(3): 505-509.
2. Chen L, Mossa-Basha M, Balu N, Canton G, Sun J, Pimentel K et al. Development of a quantitative intracranial vascular features extraction tool on 3D MRA using semiautomated open-curve active contour vessel tracing. Magnetic Resonance in Medicine 2018; 79(6): 3229-3238
3. Arteaga DF, Strother MK, Davis LT, Fusco MR, Faraco CC, Roach BA et al. Planning-free cerebral blood flow territory mapping in patients with intracranial arterial stenosis. Journal of Cerebral Blood Flow & Metabolism 2017; 37(6): 1944-1958.
4. Mutsaerts HJMM, van Dalen JW, Heijtel DFR, Groot PFC, Majoie CBLM, Petersen ET et al. Cerebral Perfusion Measurements in Elderly with Hypertension Using Arterial Spin Labeling. PLOS ONE 2015; 10(8): e0133717.
5. Xiong Y, Zhang Z, He L, et al. Intracranial simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) MRA: Analyzation, optimization, and extension for dynamic MRA. Magnetic resonance in medicine 2019;82(5):1646-1659.
Figures