0205
Clinical-related connectivity features define three biotypes of Parkinson’s disease1Department of Radiology, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China, 2Department of Neurology, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China
Synopsis
We establish brain connectivity features that represented the disease signatures and identify Parkinson’s disease (PD) subtypes by data-driven approaches. Canonical correlation analysis (CCA) was performed to define the clinical related connectivity features, which were then used in hierarchical cluster analysis to identify the distinct biotypes of PD. Multimodal MRI including gray matter functional connectivity and white matter microstructure were further used to explore the neuropsychological significance of these biotypes. CCA revealed two significant clinical-related patterns in PD. Hierarchical cluster analysis identified three neurophysiological biotypes: mild, progressive depression-dominant and progressive motor-dominant. These three biotypes characterized by different neural substrate.
Introduction
Parkinson’s disease (PD) has been recognized as a complex and heterogeneous disorder that encompasses the classic motor features and various non-motor manifestations1. Recent telencephalization pathogenesis hypothesis of PD emphasized the synchronicity of motor and non-motor symptoms and predicted the parallel manifestation of these symptoms2, implied that PD is a systematic disorder that should be assessed in a comprehensive perspective combining motor and non-motor symptoms together. Furthermore, PD Patients varies in clinical manifestations and prognosis, which point at the existence of subtypes. Identification of distinct PD subtypes are needed to better illustrate the underlying pathophysiology and predict the disease course. Aim of this study was to establish brain connectivity features that represented the disease signatures and identify PD subtypes by data-driven approaches.Methods
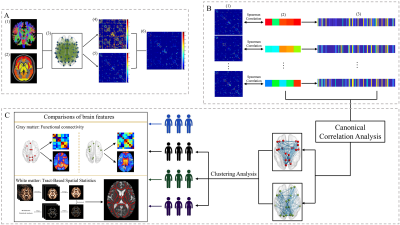
A total of 134 PD patients and 77 normal controls (NCs) who underwent both structural T1 scanning and DTI scanning were enrolled. Basic demographic information and neurologic and psychiatric scales including motor, cognition, depression, anxiety and sleep were obtained from all patients. An integrated score, global composite outcome (GCO), was calculated to evaluate the overall disease severity of PD.Data was processed using pipeline detailed in Fig. 1. Fiber connectivity matrix was constructed in each PD patients. Canonical correlation analysis (CCA) was performed to define the clinical related connectivity features, which were then used in the hierarchical cluster analysis to identify the distinct biotypes of PD. Multimodal MRI including gray matter functional connectivity and white matter microstructure were further used to explore the neuropsychological significance of these biotypes.
Results
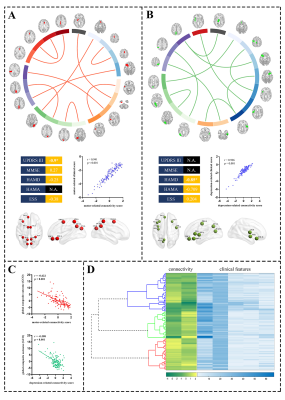
Clinical-related fiber connectivity patterns in PDCCA revealed two significantly modes that relates sets of fiber connectivity features to sets of clinical symptom measures (Fig. 2A, B). The first mode defined a connectivity pattern predominantly related to brain nodes close to the midline, and that was correlated with most clinical symptoms especially motor symptom. We termed this CCA mode as motor-related pattern. The second mode defined a set of connectivity features predominantly related to lateral limbic nodes, and that was especially related with depression. We called this CCA mode as depression-related pattern.
Clinical-related fiber connectivity pattern defines three biotypes of PD
Hierarchical clustering revealed three clusters in PD patients (Fig. 2D). Analyzing of clinical data showed the three clusters exhibited different clinical profiles. We termed these three clusters as “mild” biotype, “progressive depression-dominant” biotype (P-depression) and “progressive motor-dominant” biotype (P-motor).
Disrupt brain function and structure in three biotypes of PD
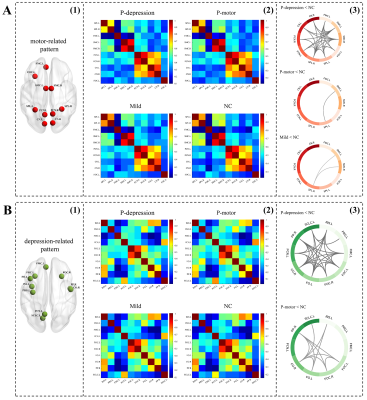
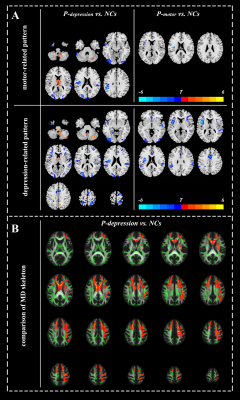
Neuroimaging analyses revealed that P-depression showed widespread disrupted functional connectivity both within motor- and depression-related connectivity pattern as well as the outside of these two patterns, covering frontal-temporal, parietal-occipital regions (Fig. 3, Fig. 4A). Noteworthy, P-depression showed widespread disruption in white matter microstructure manifested as increased MD in white matter skeleton involving superior longitudinal fasciculus, corona radiate, corpus callosum, forceps minor and uncinate fasciculus (Fig 4B).
Discussion
In the present study, we aimed to establish brain connectivity patterns that were associated with disease features, explore heterogeneous PD biotypes using unbiased objective data-driven approach and further reveal the potential structural and functional underpinnings behind these PD biotypes. The main findings were as follows: (1) motor-related and depression-related fiber connectivity patterns were observed in PD, both of which were correlated with overall disease severity (GCO score); (2) unsupervised clustering analysis using objective connectivity features defined three biotypes of PD with very different clinical profiles; (3) the P-depression biotype showed widespread disruptions both in function and structure while the other two biotypes exhibited relatively mild brain abnormalities in functional connectivity.We considered motor and non-motor symptoms together and extracted two clinical-related patterns that were most associated with motor symptoms and depression symptoms respectively. In the clinical practice, motor impairments has been recognized as a prominent component of PD and is a core criterion for PD diagnosis3, which place an emphasis on the motor disturbance in the clinical profile of PD. Moreover, PD evolves into a multi-system disorder that accompanied by a wide variety of non-motor symptoms4. Depression, a common non-motor symptom, could be appeared in the prodromal phase of PD and has been shown to nearly double an individual’s risk of subsequently developing PD5, which implied the importance of depression symptoms in PD. In our study, we extracted depression-related pattern from various non-motor symptoms, which further demonstrated that depression was an overriding manifestation beyond other non-motor symptoms in PD.
To reveal the neural basis underlying these distinct biotypes, we used multimodal MRI to detect the alterations of local and global functional connectivity for the clinical-related connectivity patterns as well as white matter microstructural among these three biotypes. Analyses of brain alterations revealed that P-depression was characterized by the extensive disruption both in gray matter functional connectivity and white matter microstructure.
Conclusion
Our study revealed the heterogeneous PD biotypes according to their distinct clinical-related connectivity patterns. Their functional and structural substrates were shown to provide significant evidences for the unsupervised clustering procedure characterized by these pattern. Importantly, predominant depression symptoms have a considerable impact on the brain damage and may exacerbate the disease progression in PD.Acknowledgements
The authors thank all the normal volunteers and Parkinson’s disease patients recruited in this project. The authors appreciate the clinical assistance from other neurologists in the Department of Neurology, the Second Affiliated Hospital of Zhejiang University School of Medicine. This work is supported by the 13th Five-year Plan for National Key Research and Development Program of China (Grant No. 2016YFC1306600), 2018 Zhejiang University Academic Award for Outstanding Doctoral Candidates.References
1.Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 2008; 79(4): 368-76.
2.Diederich NJ, James Surmeier D, Uchihara T, Grillner S, Goetz CG. Parkinson's disease: Is it a consequence of human brain evolution? Mov Disord 2019; 34(4): 453-9.
3.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992; 55(3): 181-4.
4.Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci 2017; 18(8): 509.
5.Noyce AJ, Bestwick JP, Silveira-Moriyama L, Hawkes CH, Giovannoni G, Lees AJ, et al. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol 2012; 72(6): 893-901.
Figures