0202
Reinterpreting Parkinson’s disease-related diffusion signal changes in substantia nigra1Center for Advanced Neuroimaging, University of California Riverside, Riverside, CA, United States, 2Neurology, Emory University, Atlanta, GA, United States, 3Bioengineering, University of California Riverside, Riverside, CA, United States
Synopsis
Parkinson’s disease is a progressive, neurodegenerative disorder characterized by asymmetrical onset of motor symptoms such as bradykinesia, rigidity, and tremor. The principal pathology in Parkinson's disease is the loss of melanized dopamine neurons in the substantia nigra pars compacta (SNpc) with iron deposited alongside this neuronal loss. Loss of SNpc neurons should remove barriers for diffusion and increase diffusivity of water molecules in regions undergoing this loss. Studies examining Parkinsonian SNpc microstructural changes using a single tensor model have yielded conflicting results. Here, we investigate PD-related microstructural changes in multiple compartment and single tensor models.
Introduction
Parkinson’s disease (PD) is a progressive, neurodegenerative disorder characterized by asymmetrical onset of motor symptoms such as bradykinesia, rigidity, and tremor. The principal pathology in PD is the loss of melanized dopamine neurons in the substantia nigra pars compacta (SNpc) with iron deposited alongside this neuronal loss1,2. Loss of SNpc neurons should remove barriers for diffusion and increase diffusivity of water molecules in regions undergoing this loss. These microstructural can be measured in vivo with diffusion MRI and measures sensitive to the degree of restricted diffusion (fractional anisotropy, FA) and the average (mean diffusivity, MD), axial (axial diffusivity, AD), or perpendicular (radial diffusivity, RD) rates of diffusion capture the rate of diffusion.Studies examining PD-related SNpc microstructural changes using a single tensor model have yielded conflicting results3-8. The inconsistency in results from the single tensor model may be due to the influence iron has on the diffusion signal3. Consistency has been increased by incorporating more advanced modeling approaches. In particular, the application of a bi-tensor model has yielded increases in measures sensitive to free water9-11 or multiple compartment modeling12. In this abstract, we investigate PD-related microstructural changes in multiple compartment and single tensor models and explore the relationship between diffusion and iron-metrics in PD.
Methods
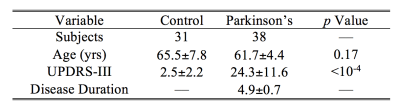
A cohort consisting of 69 subjects (31 control and 38 PD subjects) were scanned in this study. Unified Parkinson’s Disease Rating Scale Part III were collected on each subject. Relationships with MRI measures were found individually for each group using Spearman’s rank correlations. All subjects gave written, informed consent and demographic data is summarized in Table 1.Data were acquired on a 3 T MRI scanner (Prisma Fit, Siemens Medical Solutions, Malvern, PA) using a 64-channel receive only coil. MP-RAGE images (echo time (TE)/repetition time (TR)/inversion time=3.02/2600/800 ms, flip angle (FA)=8°, voxel size=0.8×0.8×0.8 mm3) were used for registration from subject space to common space.
Multi-echo data were collected with an eight echo 3D gradient recalled echo (GRE) sequence: TE1/∆TE/TR=4.92/4.92/50 ms, FOV=220×220 mm2, matrix size of 448×336×80, slice thickness=1mm, acceleration factor=2. R2* values were estimated in MATLAB by fitting a monoexponential model.
Diffusion MRI data were collected with a single-shot spin-echo, EPI sequence. Monopolar diffusion-weighting gradients were applied in 98 directions with two b values of 1000 and 2000 s/mm2; TE/TR=98.2/3230 ms, FOV=210×210 mm2, matrix size=140×140, voxel size=1.5×1.5×1.5 mm3, multiband factor=4, 92 slices with no gap, covering the entire brain. Two sets of diffusion-weighted images with phase-encoding directions of opposite polarity were acquired to correct for susceptibility distortion13-14. For each diffusion-weighted acquisition, six images without diffusion weighting (b0 images) were also acquired. DTI data were corrected for eddy current and susceptibility distortions using eddy in FSL. Neurite orientation and dispersion density imaging (NODDI) metrics were calculated in MATLAB using the NODDI toolbox v1.0.1.
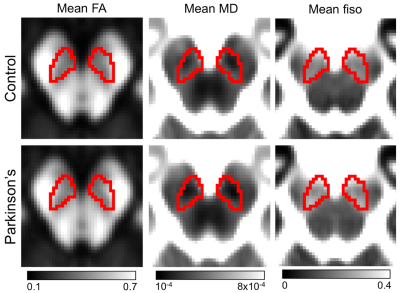
Standard space SNpc regions of interest (ROIs), made from neuromelanin-sensitive images6, were transformed from standard space to subject space using FSL. Mean R2*, DTI measures of mean diffusivity (MD), axial diffusivity (AD), radial diffusivity (RD), and fractional anisotropy (FA), as well as NODDI measures of CSF volume fraction (fiso), also referred to as the free water compartment, were calculated in the SNpc ROI for each subject.
Results
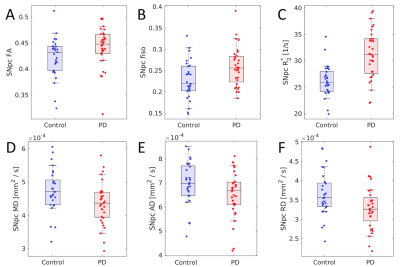
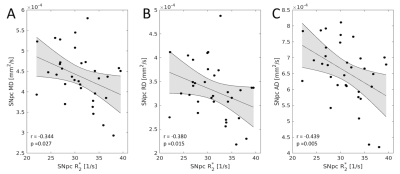
Statistically significant decreases in mean SNpc MD (p=0.02) and RD (p=0.03) were observed in the PD group relative to the control group. While trends toward increased SNpc FA (p=0.06) and decreased AD (p=0.06) were seen in the PD group. The PD group showed increased mean SNpc R2* (p<10-4) and mean SNpc fiso (p=0.005) as compared to the control group.No correlation was seen between mean SNpc R2* and fiso (r=-0.002;p=0.495). However, strong negative correlations were seen between mean SNpc R2* and diffusivity (MD: r=-0.344,p=0.027; RD: r=-0.380,p=0.015; AD: r=-0.439,p=0.005), indicating that diffusivity tends to decrease as R2* increases. An analysis of covariance (ANCOVA) was performed to remove the influence of R2* and evaluate PD-related microstructural changes in SNpc. No significant effects were observed in the ANCOVA analysis for AD (p=0.952), RD (p=0.289), or MD (p=0.544).
Discussion
The free water compartment in SNpc was found to increase in PD and this result agrees with earlier work9-12. Increases in the free water compartment are generally interpreted as a reduction of of SNpc neuronal density11 and we would expect there to be a corresponding increase in diffusivity. Paradoxically, measures related to diffusivity were found to decrease in SNpc of PD patients and this diffusivity decrease has been observed in an earlier study using SNpc ROIs derived from neuromelanin-sensitive MRI6.The reduction in diffusivity may be due to the influence of iron. In particular, iron-related measures were found to correlate negatively with diffusivity. Similar correlations have been seen in the striatum15-16 and deposits of iron create local magnetic field gradients, which produce cross terms affecting monopolar diffusion encoding gradients and reduce the apparent diffusion coefficient17-18. Thus, the conflicting results seen with the single tensor model3-8 may be attributed to two competing influences: a reduction in neuronal density, which tends to drive up diffusivity, and iron, which tends to drive diffusivity down. Future studies will need to account for the influence of iron when examining PD-related changes in SNpc microstructure.
Acknowledgements
This work received support from the Michael J. Fox Foundation (MJF 10854) as well as from NIH-NINDS 1K23NS105944-01A1.References
[1] Fearnley & Lees. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114:2283-2301.
[2] Dexter, et al. Alteration in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain 114:1953-1975.
[3] Schwarz, et al. Diffusion tensor imaging of nigral degeneration in Parkinson's disease: A region-of-interest and voxel-based study at 3 T and systematic review with meta-analysis. Neuroimage Clin 3:481-488.
[4] Peran, et al. Magnetic resonance imaging markers of Parkinson's disease nigrostriatal signature. Brain 133:3423-3433.
[5] Vaillancourt, et al. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology 72:1378-1384.
[6] Langley, et al. Diffusion tensor imaging of the substantia nigra in Parkinson's disease revisited. Hum Brain Mapp 37:2547-2556.
[7] Aquino, et al. Substantia nigra in Parkinson's disease: a multimodal MRI comparison between early and advanced stages of the disease. Neurol Sci 35 (5):753-758.
[8] Du, et al. Combined R2* and diffusion tensor imaging changes in the substantia nigra in Parkinson's disease. Mov Disord 26:1627-1632.
[9] Ofori, et al. Increased free water in the substantia nigra of Parkinson's disease: a single-site and multi-site study. Neurobiol Aging 36:1097-1104.
[10] Planetta, et al. Free-water imaging in Parkinson's disease and atypical parkinsonism. Brain 139:495-508.
[11] Arribarat, et al. Substantia nigra locations of iron-content, free-water and mean diffusivity abnormalities in moderate stage Parkinson's disease. Parkinsonism Relat Disord 65:146-152.
[12] Kamagata, et al. Neurite orientation dispersion and density imaging in the substantia nigra in idiopathic Parkinson disease. Eur Radiol 26 (8):2567-2577.
[13] Andersson, et al. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage 20, 870-888.
[14] Andersson and Sotiropoulos. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125, 1063-1078.
[15] Pfefferbaum, et al. Diffusion tensor imaging of deep gray matter brain structures: effects of age and iron concentration. Neurobiol Aging 31, 482-493.
[16] Syka, et al. Correlation between relaxometry and diffusion tensor imaging in the globus pallidus of Huntington's disease patients. PLoS One 10, e0118907.
[17] Novikov, et al. Effects of mesoscopic susceptibility and transverse relaxation on diffusion NMR. J Magn Reson 293, 134-144.
[18] Zhong, et al. Effects of susceptibility variations on NMR measurements of diffusion. J Magn Reson 95, 267-280.
Figures