0201
Introducing Substantia Nigra Iron Content and Neuromelanin Overlap to Distinguish Parkinson’s Patients from Healthy Controls1Radiology, Ruijin Hospital, Shanghai Jiaotong Univ. School of Medicine, Shanghai, China, Shanghai, China, 2Department of Radiology, Wayne State University, Detroit, MI, United States, 3Department of Biomedical Engineering, Wayne State University, Detroit, MI, United States, 4Department of Neurology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 5Magnetic Resonance Innovations, Inc., Bingham Farms, Bingham Farms, MI, United States, 6Philips Healthcare, Shanghai, China
Synopsis
A total of 40 Parkinson’s disease (PD) patients and 40 age- and sex-matched healthy controls (HC) were scanned using a single 3D gradient echo magnetization transfer sequence to measure neuromelanin and iron content, and the overlap between them. These measures showed reliable results indicative of powerful diagnostic biomarkers to differentiate PD patients from HCs. An increase in iron content was seen in the substantia nigra for the PD patients while the neuromelanin volume decreased. The best predictor, however, was found to be the combination of neuromelanin volume and its overlap with iron-containing substantia nigra which yielded an AUC of 98%.
Introduction
Diagnosing Parkinson’s disease (PD), one of the fastest growing neurodegenerative disorders1, is still challenging nowadays. There has been a lot of interests in using magnetic resonance imaging to study biomarkers of PD due to its availability and relatively low cost. Iron and neuromelanin (NM) content are known as promising biomarkers of PD. The former has been studied using quantitative susceptibility mapping (QSM) and the latter with magnetization transfer contrast (MTC). The purpose of this study was to incorporate these measures into a single susceptibility weighted imaging (SWI) MTC sequence and assess the iron content, NM content and the overlap between these two volumes as a new pathophysiological biomarker that could be used to diagnose PD.Methods
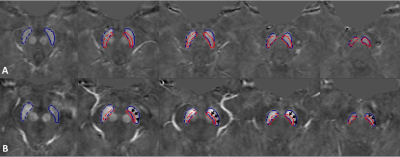
A total of 40 PD patients (age range 64.5 ± 8.4 years old) and 40 age- and sex-matched healthy controls (age range 64.2 ± 8.2 years old) were imaged on a 3T Philips scanner using a 3D multi-echo gradient echo SWI sequence with an activated MTC pulse. The imaging parameters included: seven echoes with TE1 = 7.5ms, ΔTE = 7.5ms, with TR = 62ms, flip angle = 30˚, pixel bandwidth = 174Hz/pixel, matrix size = 384 × 384, slice thickness = 2mm, and an original spatial in-plane resolution = 0.67 × 1.34mm2 then interpolated to 0.67 × 0.67mm2. The first echo in the MTC-SWI magnitude image (TE = 7.5ms) was used to delineate the NM content in the midbrain. The second echo (TE = 15ms) was used for QSM analysis. The susceptibility maps were created using the following steps: the brain extraction tool, BET, to isolate the brain tissue2, a 3D phase unwrapping algorithm (3DSRNCP) to unwrap the original phase data3, sophisticated harmonic artifact reduction (SHARP) to remove unwanted background fields4, and a truncated k-space division (TKD) based inverse filtering technique5 with an iterative approach to reconstruct the final QSM maps.6 The regions of interest (ROIs) for the NM and substantia nigra (SN) were manually drawn on MTC magnitude and QSM maps, respectively, using SPIN software (SpinTech, Inc., Bingham Farms, MI, USA). Iron deposition in the SN was evaluated using the susceptibility maps generated from the MTC-SWI phase data. To evaluate the overlap between putative NM content and iron deposition in the SN, the ROIs from the MTC-SWI magnitude images were superimposed on those of the SN from the corresponding MTC-QSM maps (Figure 1) and normalized to the SN volume, known as the overlap fraction. Furthermore, in order to assess the sensitivity and specificity of the proposed models to distinguish between the HC and PD cases, a receiver operating curve (ROC) and area under the curve (AUC) analysis were performed on different parameters using IBM SPSS Statistics Version 22.0.Results
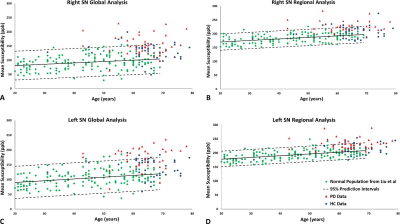
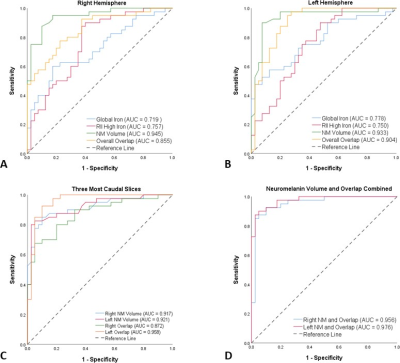
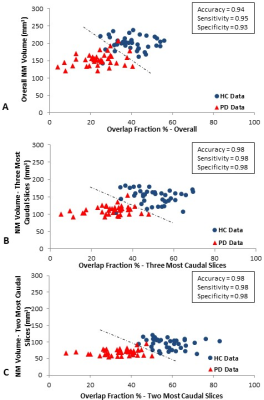
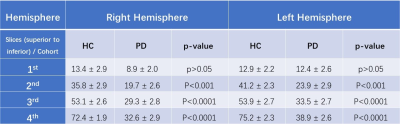
Figure 2 shows the data associated with the global and regional analyses superimposed on the corresponding susceptibility-age baselines, established by Liu et al.7 The AUCs from the ROC curves were calculated as 0.719 and 0.757 for the right hemisphere and 0.778 and 0.750 for the left hemisphere, for the global and regional analyses, respectively. Table 1 contains the values of the overlap fraction for the four most caudal slices where both structures were visible. The overlap fraction in both HC and PD appears to increase from cranial to caudal slices. However, in the three most caudal slices, a significant decrease is observed in the overlapping regions in the PD cohort compared to those of the HC cohort. Figure 3 illustrates the ROC curves associated with overall NM volume, mean iron content and the overall overlap fraction in both hemispheres, in three most caudal slices, as well as the combination of NM volume and the overall overlap fraction, which was calculated using binary logistic regression. The NM volume and overlap fraction showed significantly larger AUCs than those of global and regional iron content in both hemispheres. The ROC curves illustrated in Figure 3-D demonstrate the effective AUC values 0.956 and 0.976 for the right and left hemisphere, respectively, generated from combining the overall NM volume and the NM/iron overlap. Figure 4 shows the NM volume against the normalized NM overlap with the iron-containing SN in the corresponding caudal slices, averaged between the right and left hemispheres, as well as the best threshold to achieve the maximum sensitivity and specificity.Discussion and conclusion
In this work, we have introduced a new approach to show iron deposition and NM degeneration and their overlap using one single multi-echo SWI MTC sequence in an attempt to develop a comprehensive pathophysiological biomarker that could be used clinically to diagnose PD. The NM volume and NM/iron overlap provide strong measures to differentiate PD patients from HCs. We have demonstrated that this diagnostic biomarker can be determined using a single rapid sequence with clinically useful diagnostic accuracy (with an AUC as high as 98%). However, a larger cohort of PD patients and HCs is required to validate the diagnostic power of this approach prior to clinical practice.Acknowledgements
No acknowledgement found.References
1. Group GBDNDC. Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 2017; 16(11): 877-97.
2. Smith SM. Fast robust automated brain extraction. Human brain mapping 2002; 17(3): 143-55.
3. Abdul-Rahman HS, Gdeisat MA, Burton DR, Lalor MJ, Lilley F, Moore CJ. Fast and robust three-dimensional best path phase unwrapping algorithm. Applied optics 2007; 46(26): 6623-35.
4. Schweser F, Deistung A, Lehr BW, Reichenbach JR. Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: an approach to in vivo brain iron metabolism? NeuroImage 2011; 54(4): 2789-807.
5. Haacke EM, Tang J, Neelavalli J, Cheng YC. Susceptibility mapping as a means to visualize veins and quantify oxygen saturation. Journal of magnetic resonance imaging: JMRI 2010; 32(3): 663-76.
6. Tang J, Liu S, Neelavalli J, Cheng YC, Buch S, Haacke EM. Improving susceptibility mapping using a threshold-based K-space/image domain iterative reconstruction approach. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2013; 69(5): 1396-407.
7. Liu M, Liu S, Ghassaban K, Zheng W, Dicicco D, Miao Y, et al. Assessing global and regional iron content in deep gray matter as a function of age using susceptibility mapping. Journal of magnetic resonance imaging: JMRI 2016; 44(1): 59-71.
Figures