0196
Increase in blood-brain barrier disruption during normal aging1Department of Psychiatry and Neuropsychology, Maastricht University, Maastricht, Netherlands, 2Department of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 3Department of Electrical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands
Synopsis
Blood-brain barrier (BBB) disruption is assumed to increase with age, but this has not been demonstrated assessing gadolinium leakage using dynamic contrast-enhanced MRI.
We determined BBB leakage rate in healthy middle-aged to elderly individuals (47 - 91 years) combining DCE MRI with pharmacokinetic modeling. Results demonstrated BBB leakage in white and gray matter increased with age. However, this effect was not independent of white matter lesions or cortical thinning, so other physiological changes may influence the age and leakage association.Our study demonstrates that BBB disruption manifests in normal aging, before the emergence of neuropathology.
Purpose
Blood-brain barrier (BBB) disruption impairs oxygen and nutrient supply and waste clearance1. Subsequent pathophysiological processes make the brain vulnerable to neuronal dysfunction2. Previous studies have supported the notion that BBB disruption could be an early event in neurodegeneration3 and cognitive dysfunction4.A large meta-analysis has demonstrated that BBB permeability increases already in normal aging5. However, in these studies the blood/CSF albumin ratio was used as biomarker for BBB leakage, which is an indirect measure that cannot localize leakage2 and relies on a biomolecule much larger than gadolinium-based contrast agent. Dynamic contrast-enhanced (DCE) MRI can be used to both detect and localize gadolinium-based contrast agent leakage over time from the blood into the interstitial fluid of the brain parenchyma. To distinguish the rapidly circulating component from the slowly extravasating component, dual-time resolution can ideally be applied. This acquisition technique has a high temporal resolution during the initial circulations and long-term sampling during the leakage phase6.
Thusfar, DCE MRI studies into BBB disruption with normal aging individuals are lacking, which hampers the value estimation and interpretation of BBB leakage observed in recent studies on brain pathology. Therefore, we aimed to investigate the association between BBB leakage and age in healthy, middle-aged to elderly individuals using DCE MRI.
Methods
57 middle-aged to elderly (mean age: 65 years, range: 47 – 91), healthy participants from the Maastricht Aging Study (MAAS)7 (Mini-Mental State Examination score ≥ 25; no diagnosis of dementia, prodromal dementia, mild cognitive impairment or other psychiatric or neurological disorders; no neuroradiological brain abnormalities; no cognitive impairment due to substance abuse) were selected for brain imaging. The anatomical scans consisted of T1-weighted fast gradient echo and T2-weighted FLAIR, turbo spin echo and gradient echo sequences acquired on a 3T MRI system (Achieva TX, Philips, Best, the Netherlands). For leakage detection a 25-min lasting dual-time resolution DCE MRI protocol was used, during which a short (3.2 s) and long (30.5 s) dynamic scan interval were alternated8. During the short interval, a bolus injection (0.1 mmol/kg gadobutrol, Gadavist®, Bayer AG, Leverkusen, Germany) was injected intravenously. T1-mapping was performed prior to contrast administration.WMH volume was determined using a semi-automatic segmentation tool (GIANT10). Whole cerebrum white matter, gray matter and hippocampal regions, from which cortical thickness and hippocampal volume were estimated, were obtained using automatic software segmentation (FreeSurfer version 6.0.011) with manual adjustments.
The patient-specific vascular input function (VIF) was derived from the superior sagittal sinus, and the graphical Patlak method9 was used for voxel-wise pharmacokinetic modeling of the contrast concentration in tissue and plasma to obtain leakage rate Ki. Histograms of the leakage rates in white matter, gray matter and hippocampi regions were corrected for noise8, after which the mean Ki was calculated for each region as indication of BBB permeability. Linear regression analyses were performed with cuberoot transformed Ki values to obtain normality.
Results
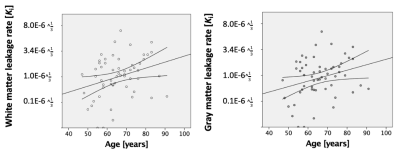
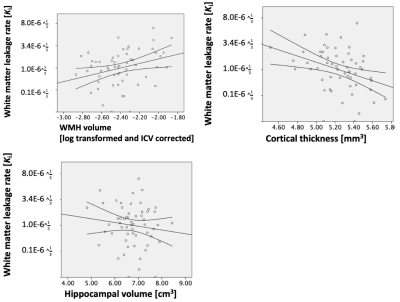
Leakage rate increased significantly with age in both white (p = .025) and gray (p = .036) matter (Figure 1).Leakage rate in the white and gray matter was positively associated with WMH volume (white matter: p = 0.020, gray matter: p = 0.032; Figure 2). After correction for WMH volume, the association between white or gray matter leakage rate and age disappeared.
Leakage rate in the white matter was negatively associated with cortical thickness (p = .007; Figure 2), and leakage rate in the gray matter approached significance (p = .051). After correction for cortical thickness, the association between white or gray matter leakage rate and age disappeared.
No association could be detected between leakage rate and hippocampal volume (Figure 2), and the association between white or gray matter leakage rate and age did not depend on hippocampal volume.
Leakage rate in the hippocampus had no significant associations with age or the other imaging variables, WHH volume, cortical thickness or hippocampal volume.
Discussion
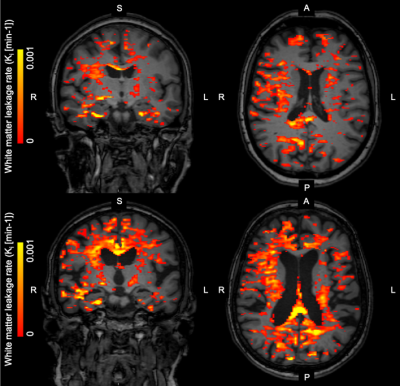
Our results demonstrated that widespread BBB leakage in the white and gray matter increased with age in healthy, middle-aged to elderly individuals. Leakage specifically located in the hippocampus showed no age effect.WMH volume increases with age12. WMHs and its nearby normal appearing white matter tissue are more permeable13, which could explain why any relation between age and BBB leakage could only be found in the brain regions with these lesions, which is rather distant to the hippocampus.
The association between leakage rate and age did not appear independent of white matter lesions or cortical thinning, which could be normal physiological aging phenomena12, 14. Thus, BBB disruption appears to increase with age and is likely a result of normal physiological aging. As BBB disruption was not found to depend on hippocampal volume loss, the latter is suggested to originate from a different pathophysiological process (e.g. neurodegeneration).
Conclusion
BBB leakage increases with age as a normal physiological process, suggesting that BBB disruption could be an early phenomenon in the aging process before overt signs of neuropathology and functional disabilities become apparent. BBB disruption could be a promising target for early diagnosis or prevention of neurodegenerative disorders.Acknowledgements
This work was supported by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) [grant number 406-15-031].References
1. Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nature Reviews Neuroscience. 2011;12:723.
2. Raja R, Rosenberg GA, Caprihan A. MRI measurements of Blood-Brain Barrier function in dementia: A review of recent studies. Neuropharmacology. 2018;134:259-71.
3. Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Pérez JM, Evans AC, The Alzheimer’s Disease Neuroimaging I, et al. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nature Communications. 2016;7:11934.
4. Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nature Medicine. 2019.
5. Farrall AJ, Wardlaw JM. Blood–brain barrier: ageing and microvascular disease–systematic review and meta-analysis. Neurobiology of aging. 2009;30(3):337-52.
6. Haar HJ, Jansen JFA, Jeukens CRLPN, Burgmans S, Buchem MA, Muller M, et al. Subtle blood-brain barrier leakage rate and spatial extent: Considerations for dynamic contrast-enhanced MRI. Medical Physics. 2017;44(8):4112-25.
7. Jolles J, Houx P, Van Boxtel M, Ponds R. Maastricht aging study: Determinants of cognitive aging: Neuropsych Publishers Maastricht; 1995.
8. van de Haar HJ, Burgmans S, Jansen JF, van Osch MJ, van Buchem MA, Muller M, et al. Blood-brain barrier leakage in patients with early Alzheimer disease. Radiology. 2016;281(2):527-35.
9. Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3(1):1-7.
10. Gronenschild EH, Burgmans S, Smeets F, Vuurman EF, Uylings HB, Jolles J. A time-saving and facilitating approach for segmentation of anatomically defined cortical regions: MRI volumetry. Psychiatry Research: Neuroimaging. 2010;181(3):211-8.
11. Fischl B, Sereno MI, Dale AM. Cortical Surface-Based Analysis: II: Inflation, Flattening, and a Surface-Based Coordinate System. NeuroImage. 1999;9(2):195-207.
12. Maniega SM, Hernández MCV, Clayden JD, Royle NA, Murray C, Morris Z, et al. White matter hyperintensities and normal-appearing white matter integrity in the aging brain. Neurobiology of aging. 2015;36(2):909-18.
13. Simpson JE, Wharton SB, Cooper J, Gelsthorpe C, Baxter L, Forster G, et al. Alterations of the blood–brain barrier in cerebral white matter lesions in the ageing brain. Neuroscience letters. 2010;486(3):246-51.
14. Thambisetty M, Wan J, Carass A, An Y, Prince JL, Resnick SM. Longitudinal changes in cortical thickness associated with normal aging. Neuroimage. 2010;52(4):1215-23.
Figures