0193
Reliable High-Resolution MR Elastography Protocol to Assess Hippocampal Subfield Viscoelasticity in Aging1University of Delaware, Newark, DE, United States, 2Wayne State University, Detroit, MI, United States, 3University of Illinois at Urbana-Champaign, Urbana, IL, United States, 4Dartmouth College, Hanover, NH, United States
Synopsis
The goal of this study is to generate the first high-resolution magnetic resonance elastography (MRE) protocol specifically for characterizing viscoelasticity of the hippocampal subfields (HCsf) and analyzing the effects of age on HCsf properties. We demonstrated that the protocol can sensitively and reliably differentiate between HCsf regions. We find that each HCsf decreases in stiffness and increases in damping ratio with age, and that HCsf exhibit differential relationships with age. This protocol shows promise for investigating the HCsf in health and disease.
Introduction
Aging is a significant risk factor in the development of neurological disorders and can lead to a loss of tissue integrity and cognitive function1,2. There is an emerging interest in measuring the viscoelasticity of the aging brain using magnetic resonance elastography (MRE), a technique used to sensitively assess tissue health3. Previous MRE studies have demonstrated that the brain, its lobes, and subcortical structures decline in viscoelasticity during aging4-6. The hippocampus (HC) typically demonstrates age-related atrophy7-9 and reveals a consistent viscoelastic structure-function relationship with memory performance10,11. The purpose of this work is to extend MRE methods to examine the viscoelasticity of the individual HC subfields (HCsf), which are cytoarchitecturally distinct and show differential sensitivity to aging and support specific memory functions12,13. The goal of this study is to establish a high-resolution MRE protocol for sensitively analyzing the HCsf and to characterize how they are affected during healthy aging.Methods
Forty-nine healthy subjects (age: 23-81y; mean: 55±17y) with no history of neurological conditions were scanned using a high-resolution MRE protocol. Three additional subjects (25-29y) were scanned four times to test the repeatability of the HCsf protocol.Subjects completed an MRI session using a 3T Siemens Prisma scanner and 64-channel head coil. A 3D multiband, multishot spiral MRE sequence14 was used to image whole-brain displacements at a 1.25 mm isotropic resolution in 10:45 total time (240x240 mm2 FOV, 96 slices, TR/TE = 3360/70 ms). We used a Resoundant pneumatic driver system to apply 50 Hz acoustic vibrations and induce brain tissue deformation. Structural scans included a T1-weighted MPRAGE scan at 0.9 mm isotropic resolution and a T2-weighted TSE scan with 0.4x0.4x2.0 mm3 resolution aligned to the hippocampus. Automated Segmentation of Hippocampal Subfields (ASHS) software with the Penn Memory Center 3T atlas was used to segment each HCsf region: DG-CA3, CA1-CA2, SUB, and ERC15. We then transformed the subfield segmentations into MRE space using FLIRT in FSL and thresholded each segment to create binary masks. Nonlinear inversion (NLI) was used to create maps of shear stiffness and damping ratio16. We included each HCsf mask as a separate region for soft prior regularization (SPR) in NLI17 (Figure 1).
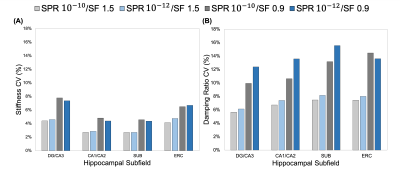
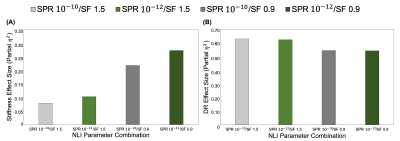
We analyzed a subset of the data with different inversion parameters to determine the ability to sensitively differentiate the mechanical properties of the HCsf through partial-η2 from a repeated measures ANOVA. The coefficient of variation (CV) from repeat measures was used to evaluate the repeatability of the protocol. We tested these measures with different combinations of SPR weightings and NLI spatial filtering (SF) widths (SPR: 10-10 and 10-12; SF: 1.5 and 0.9 mm)17. A linear mixed model with age and HCsf group was used to analyze how stiffness and damping ratio differed between HCsf and with age. We characterized each individual HCsf with age using a second order relationship4. An age x HCsf group interaction was tested to determine if the HCsf exhibited different relationships with age.
Results
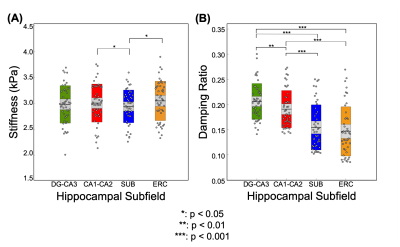
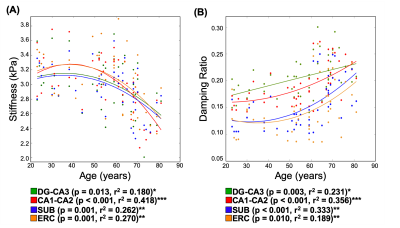
The repeatability of the HCsf protocol demonstrated that stiffness CVs did not strongly change with parameter combinations, while damping ratio exhibited higher CVs with reduced spatial filtering (Figure 2). The sensitivity analysis demonstrated that the stiffness effect size improved with reduced spatial filtering, while the damping ratio effect sizes were consistent across parameter combinations (Figure 3).Analysis of the entire sample revealed that there were significant differences between the HCsf regions for both stiffness (F(3,46.4)=4.45, p=0.008) and damping ratio (F(3,139.7)=10.9, p<0.001). Post-hoc tests with Bonferroni correction revealed significant individual differences between individual HCsf (Figure 4). All HCsf exhibited a decrease in stiffness and an increase in damping ratio with age (Figure 5). There was a significant age x HCsf interaction effect in damping ratio (F(3,45.2)=4.047, p=0.012), indicating the subfields exhibit different relationships with age. There was no significant interaction for stiffness (F(3,47)=1.059, p=0.375).
Discussion and Conclusions
We tailored an MRE protocol to assess the viscoelasticity of the small HCsf by combining displacement data at a high spatial resolution and tuning NLI parameters to optimize protocol performance. HCsf stiffness estimates were highly repeatable for all parameter combinations, while reduced spatial filtering greatly improved sensitivity for differentiating subfields. Conversely, the damping ratio was highly sensitive to subfield group regardless of NLI parameters, though reducing filtering negatively impacted repeatability. This analysis suggests an optimal protocol uses two inversions: reduced filtering for HCsf stiffness to improve sensitivity and greater filtering for HCsf damping ratio to improve stability.This study demonstrates the ability of the high-resolution MRE protocol to reliably measure the viscoelasticity of individual HCsf. We can mechanically distinguish the individual HCsf, which is expected given their cytoarchitectural differences12,13. HCsf viscoelasticity follows a similar pattern of age-related decline that is sharper in the older age population (>50) as in previous whole-brain studies4,5. Furthermore, we showed a significant interaction between age and HCsf for the damping ratio measure, indicating that the HCsf exhibit different relationships with age. Future work involves collecting longitudinal aging measurements to confirm these cross-sectional findings, correlating HCsf viscoelastic measures with memory performance, and using HCsf properties as clinical measures to characterize differential effects in neurological conditions, such as mild cognitive impairment and Alzheimer’s disease.
Acknowledgements
This project was supported by the National Institutes of Health (R01-AG058853), Delaware INBRE (P20-GM103446), Delaware Cardiovascular COBRE (P20-GM113125), Delaware Neuroscience COBRE (P20-GM103653), University of Delaware Research Foundation, and Delaware Rehabilitation Institute.References
[1] López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6), 1194-1217.
[2] Lindsay, J., Laurin, D., Verreault, R., Hébert, R., Helliwell, B., Hill, G. B., & McDowell, I. (2002). Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. American journal of epidemiology, 156(5), 445-453.
[3] Hiscox, L. V., Johnson, C. L., Barnhill, E., McGarry, M. D., Huston 3rd, J., Van Beek, E. J., Starr JM., & Roberts, N. (2016). Magnetic resonance elastography (MRE) of the human brain: technique, findings and clinical applications. Physics in Medicine & Biology, 61(24), R401.
[4] Sack, I., Streitberger, K. J., Krefting, D., Paul, F., & Braun, J. (2011). The influence of physiological aging and atrophy on brain viscoelastic properties in humans. PloS one, 6(9), e23451.
[5] Arani, A., Murphy, M.C., Glaser, K.J., Manduca, A., Lake, D.S., Kruse, S.A., Jack Jr, C.R., Ehman, R.L. & Huston 3rd, J. (2015). Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. Neuroimage, 111, 59-64.
[6] Hiscox, L.V., Johnson, C.L., McGarry, M.D., Perrins, M., Littlejohn, A., Van Beek, E.J., Roberts, N. & Starr, J.M. (2018). High-resolution MR Elastography (MRE) reveals differences in Subcortical Gray Matter Viscoelasticity between Young and Healthy Older Adults. Neurobiology of Aging, 65, 158-167.
[7] Small, S. A., Chawla, M. K., Buonocore, M., Rapp, P. R., & Barnes, C. A. (2004). Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proceedings of the National Academy of Sciences, 101(18), 7181-7186.
[8] Du, A.T., Schuff, N., Chao, L.L., Kornak, J., Jagust, W.J., Kramer, J.H., Reed, B.R., Miller, B.L., Norman, D., Chui, H.C. & Weiner, M.W. (2006). Age effects on atrophy rates of entorhinal cortex and hippocampus. Neurobiology of aging, 27(5), 733-740.
[9] Petersen, R.C., Jack, C.R., Xu, Y.C., Waring, S.C., O’brien, P.C., Smith, G.E., Ivnik, R.J., Tangalos, E.G., Boeve, B.F. & Kokmen, E. (2000). Memory and MRI-based hippocampal volumes in aging and AD. Neurology, 54(3), 581-581.
[10] Schwarb, H., Johnson, C. L., McGarry, M. D., & Cohen, N. J. (2016). Medial temporal lobe viscoelasticity and relational memory performance. Neuroimage, 132, 534-541.
[11] Schwarb, H., Johnson, C. L., Daugherty, A. M., Hillman, C. H., Kramer, A. F., Cohen, N. J., & Barbey, A. K.
[12] Mueller, S. G., Stables, L., Du, A. T., Schuff, N., Truran, D., Cashdollar, N., & Weiner, M. W. (2007). Measurement of hippocampal subfields and age-related changes with high resolution MRI at 4 T. Neurobiology of aging, 28(5), 719-726.
[13] Daugherty, A. M., Bender, A. R., Raz, N., & Ofen, N. (2016). Age differences in hippocampal subfield volumes from childhood to late adulthood. Hippocampus, 26(2), 220-228. (2017). Aerobic fitness, hippocampal viscoelasticity, and relational memory performance. Neuroimage, 153, 179-188.
[14] CL Johnson, JL Holtrop, AT Anderson, BP Sutton, “Brain MR Elastography with Multiband Excitation and Nonlinear Motion-Induced Phase Error Correction,” 24th Annual Meeting of the International Society for Magnetic Resonance in Medicine, Singapore, May 7-13, 2016, p. 1951.
[15] Yushkevich, P.A., Pluta, J.B., Wang, H., Xie, L., Ding, S.L., Gertje, E.C., Mancuso, L., Kliot, D., Das, S.R. & Wolk, D.A. (2015). Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Human brain mapping, 36(1), 258-287.
[16] McGarry, M. D. J., Van Houten, E. E. W., Johnson, C. L., Georgiadis, J. G., Sutton, B. P., Weaver, J. B., & Paulsen, K. D. (2012). Multiresolution MR elastography using nonlinear inversion. Medical physics, 39(10), 6388-6396.
[17] McGarry, M., Johnson, C. L., Sutton, B. P., Van Houten, E. E., Georgiadis, J. G., Weaver, J. B., & Paulsen, K. D. (2013). Including spatial information in nonlinear inversion MR elastography using soft prior regularization. IEEE transactions on medical imaging, 32(10), 1901-1909.
Figures