0192
Age differences in hippocampal glutamate modulation during associative encoding: A proton functional magnetic resonance spectroscopy study1Psychiatry and Behavioral Neuroscience, Wayne State University, Detroit, MI, United States, 2Psychology, Wayne State University, Institute of Gerontology, Detroit, MI, United States
Synopsis
Memory declines early in normal aging, worsening in dementia. Glutamatergic neurons, abundant in the hippocampus, play a pivotal role in synaptic plasticity underlying formation of associations. We have previously demonstrated with 1H fMRS, significant modulation of hippocampal glutamate during encoding of object-location associations. We observed that the timing of modulation differentiated proficiency in acquiring the associations. Because age-related hippocampal atrophy may be accompanied by glutamatergic dysfunction, age-differences in task-related levels of hippocampal glutamate may provide a marker of age-related memory deficits. Here, we identified age-differences in hippocampal glutamate modulation during associative memory encoding, which may underlie age-related associative memory deficits.
Introduction
Although age-related cognitive declines are common in healthy adults; in some they devolve into dementia (e.g. Alzheimer disease, AD)1. On a continuum of age-related decline, the AD neuropathology precedes cognitive changes by years2, which makes the identification of biomarkers tracking early stages of disease progression important3. Episodic (e.g., associative) memory decline is present early in normal aging4 as well as in dementias1. Age-related deficits in associative memory have been explained by the associative deficit hypothesis stating that the elderly are impaired in binding disparate pieces of information during the encoding stage of memory4. Hippocampal glutamate plays a central role in forming associations6. The hippocampus is particularly rich in glutamatergic neurons that are crucial for memory acquisition and maintenance7,8,9. Understanding age-related glutamatergic dysfunction5 may therefore, help track early decline in cognition. We have previously demonstrated in healthy young adults, a significant increase in hippocampal glutamate during associative memory encoding and highlighted the link between the timing of glutamate modulation during encoding and rate of acquisition of associations10,11. The current 1H fMRS study investigates the role of glutamate in age-related differences in memory for object-location associations, and is ultimately expected to contribute to the search for new functional neural biomarkers of age-related cognitive decline that will help develop better-targeted therapies.Methods
Healthy young (n = 29, 16 females, mean age 25.2 ± 2.4) and older (n = 16, 8 females, mean age 65.6 ± 2.7) adults participated in a morning ¹H fMRS scan on a 3T Siemens Verio system. Following a structural T1-weighted scan (MPRAGE), a single-voxel (30 x 18 x 13 mm) was manually prescribed in a randomly selected right or left hippocampus (Figure 1), after which B0-field shimming using FASTESTMAP was performed. During the ¹H fMRS, participants performed, in the scanner, an object-location associative memory task. Each run included epochs of encoding (12 unique object-location pairs; 32 s) and retrieval (32 s) interspersed by epochs of counting aloud backwards (16 s) to block memory rehearsal. Twelve encoding-counting-retrieval-counting cycles were employed to allow learning to asymptote. The task also included a 2 Hz flashing-checkerboard condition at the beginning (5 min). A total of 92 1H MRS measurements were acquired every 16 s (PRESS with OVS and VAPOR, TE = 23 ms, TR = 2670 ms, 6 averages/measurement; 2048 data points; scan time 24:30 min). In addition, 20 ¹H MRS measurements during a fixation-crosshair were acquired as a separate scan to serve as the non-task-active control condition. To increase the signal to noise ratio of the ¹H MRS signal, measurements at three consecutive epochs of each task condition were phased (0th and 1st order) and frequency shift corrected prior to averaging and quantification using LCModel (v6.3) (Figure 2A and 2B). Performance was modeled by fitting the number of correct paired-object locations over time (epoch number) to a three-parameter Gompertz function characterized by learning rate, asymptote, and inflection point. The primary outcome variables of interest were glutamate levels and percent glutamate modulation during encoding and retrieval with respect to the control condition. The statistical analyses assessed age-group differences in glutamate modulation during the encoding and retrieval as well as age-group-by-time (epoch number) interactions for glutamate for both task conditions (encoding, retrieval). In these analyses, we used the repeated measure GEE approach. Descriptive data are presented as mean ± 1 standard error (SE) unless otherwise specified.Results
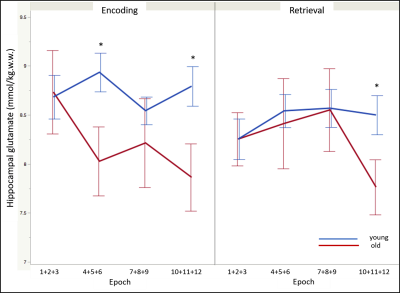
Regarding memory performance, the asymptote was the only parameter that differed between the age-groups and was significantly lower in the older participants [F(1, 43) = -11.52 , p < .05]. The glutamate level acquired during the control condition was non-significantly lower in the older compared to young adults [t(43) = -1.39, p = .08]. During memory encoding and independent of time, old adults demonstrated significantly lower glutamate levels compared to the younger counterparts [t(43) = -2.05, p < .05]. In contrast, no age-differences on glutamate modulation were observed during retrieval. The temporal dynamics of glutamate modulation significantly differed between age groups during encoding (age-group x epoch: F(3, 129) = 3.42, p < .05). Post-hoc analyses revealed lower glutamate modulation in the older adults during epochs 4 (average of encoding epochs 4, 5, and 6) (p < .002) and 10 (average of encoding epochs 10, 11, and 12) (p < .002); and during epoch 10 of retrieval (p < .002) (Figure 3).Conclusion
Older adults performed significantly worse on the associative memory task compared to their younger counterparts. Significant age-differences on the hippocampal modulation of glutamate observed overall, and across time, were specific to the encoding stage, with the older adults demonstrating lower and delayed glutamate modulation. Retrieval did not evince age-differences in glutamate modulation. This agrees with the associative deficit hypothesis4 that posits that older adults experience impairments in binding items into a cohesive memory representation, thus confirming the sensitivity of the memory encoding processes to aging. It is plausible, therefore, that deficits in glutamate modulation specific to memory encoding may underlie age-related memory decline.Acknowledgements
The authors acknowledge Caroline Zajac-Benitez and Asadur Chowdury for their administrative and technical support.
The authors acknowledge the following funding sources: NIH F31-AG058420 (CA); NIH R21-AG059160 (JAS and NR); The Lycaki-Young Funds from the State of Michigan
References
- A. M. Fjell, L. McEvoy, D. Holland, A. M. Dale, K. B. Walhovd, and I. Alzheimer's Disease Neuroimaging. What is normal in normal aging? Effects of aging, amyloid and Alzheimer's disease on the cerebral cortex and the hippocampus. Prog Neurobiol, 2014, 117, 20-40.
- N. Raz, U. Lindenberger, K. M. Rodrigue, K. M. Kennedy, D. Head, A. Williamson, C. Dahle, D. Gerstorf, and J. D. Acker. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex, 2005, 15(11), 1676-89.
- L. Schneider. Alzheimer's disease and other dementias: update on research. Lancet Neurol, 2017, 16(1), 4-5.
- M. Naveh-Benjamin. Adult age differences in memory performance: tests of an associative deficit hypothesis. J Exp Psychol Learn Mem Cogn, 2000, 26(5), 1170-87.
- R. Rupsingh, M. Borrie, M. Smith, J. L. Wells, and R. Bartha. Reduced hippocampal glutamate in Alzheimer disease. Neurobiol Aging, 2011, 32(5), 802-10.
- W. A. Suzuki. Making new memories: the role of the hippocampus in new associative learning. Ann N Y Acad Sci, 2007, 1097, 1-11.
- D. A. Butterfield and C. B. Pocernich. The glutamatergic system and Alzheimer's disease: therapeutic implications. CNS Drugs, 2003, 17(9), 641-52.
- C. J. Tabone and M. Ramaswami. Is NMDA receptor-coincidence detection required for learning and memory? Neuron, 2012, 74(5), 767-9.
- W. J. McEntee and T. H. Crook. Glutamate: its role in learning, memory, and the aging brain. Psychopharmacology (Berl), 1993, 111(4), 391-401.
- J. Stanley, A. Burgess, D. Khatib, K. Ramaseshan, M. Arshad, H. Wu, and V. Diwadkar. Functional dynamics of hippocampal glutamate during associative learning assessed with in vivo 1H functional magnetic resonance spectroscopy. Neuroimage, 2017, 153: 189-197.
- J. Stanley and N. Raz. Functional Magnetic Resonance Spectroscopy: The "New" MRS for Cognitive Neuroscience and Psychiatry Research. Front Psychiatry, 2018: 9(76), 1-12.
Figures
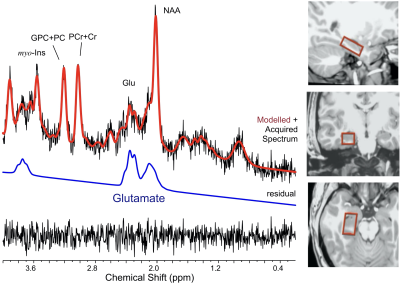
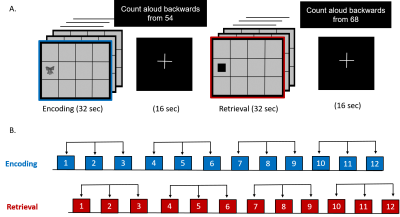
Figure 2(A): Associative learning and memory task involving epochs of encoding (12 unique object-location pairs), cued-retrieval of associated memoranda, interspersed with counting backwards epochs.
Figure 2(B): Three consecutive epochs of encoding and retrieval were averaged to improve the signal to noise ratio.