0188
Association of age and sex with cerebral blood flow measured using pseudo-continuous arterial spin labeling imaging1NIA, NIH, Baltimore, MD, United States
Synopsis
Cerebral
blood flow (CBF) has been shown to decline with age and differs between men and
women. However, limited work has been conducted on cognitively unimpaired
subjects. Furthermore, most investigations focus on gray matter (GM), with few results
reported for white matter (WM), in which CBF is lower and represents a particularly
challenging measurement. We investigate associations of age and sex with CBF in
GM and WM regions in a cohort of cognitively unimpaired subjects across a wide
age range. We find significant correlations
between CBF and age, as well as sexual dimorphism of CBF, in critical brain
structures.
PURPOSE
Our main goals are to characterize the regional differences between cerebral blood flow (CBF) in white matter (WM) and gray matter (GM), measured using the pseudo-continuous arterial spin labeling (pCASL) technique (1), and age and sex in a cohort of cognitively unimpaired subjects across a wide age range, and to provide reference values for CBF in critical WM and GM structures.METHODS
Subjects and MRIOur study cohort consisted of 77 cognitively unimpaired participants (48.8 ± 19.0 years, age range of 22-88 years) incorporating 30 women (47.4 ± 18.9 years) and 47 men (49.7 ± 19.2 years). Age was not statistically different between men and women. For each participant, pCASL imaging datasets for CBF mapping were acquired (1). Datasets consisted of control, labeled, and proton density (PD) images that were acquired with incorporation of background suppression, FoV of 220 mm x 210 mm x 120 mm, and spatial resolution of 2.5 mm x 2.5 mm x 5 mm with reconstruction to 1 mm x 1 mm x 1 mm. Other experimental parameters were: TE of 15 ms, TR of 7.5 s, labeling duration of 1.8 s, post-labeling duration of 2 s, and 30 signal averages.
Image processing and statistical analysis
For each participant, a whole-brain CBF map was generated from the pCASL dataset using the NESMA analysis to improve accuracy and precision in CBF determination (2). The PD image was nonlinearly registered to the MNI space and the computed transformation matrix was then applied to the corresponding CBF map. FAST segmentation was performed on PD images to generate WM masks and cortical GM masks. Six WM and GM regions of interest (ROIs) were defined from the MNI structural atlas using the FAST-derived WM and GM masks; these ROIs correspond to the whole brain, and the frontal, parietal, temporal and occipital lobes, as well as the cerebellum. Within each WM and GM ROI, the mean CBF value was calculated. The registration, segmentation, and ROI analyses were performed using FSL software (3). Finally, for each ROI, the effect of CBF was investigated using linear regression with the mean CBF value within the ROI as the dependent variable and sex and age as the independent variables.
RESULTS & DISCUSSION
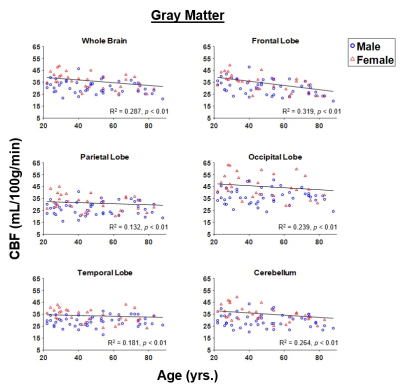
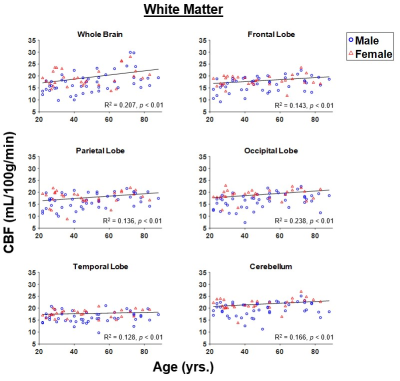
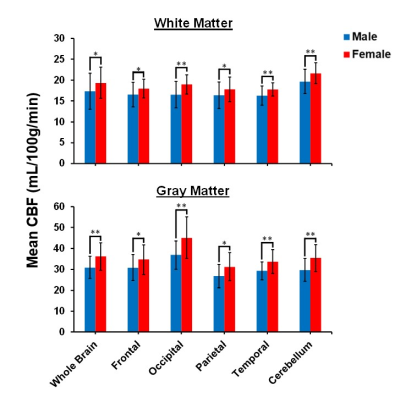
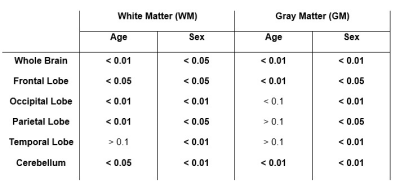
Figure 1 shows representative CBF maps derived from pCASL imaging datasets. These NESMA-filtered images exhibited excellent detail (2). Results are shown for three slices covering the main brain structures and for seven participants of different ages. Visual inspection of CBF maps indicate an overall decrease in CBF with age, especially in the GM brain regions. Regression analysis quantified these changes in CBF in GM (Fig. 2) and WM (Fig. 3) regions. As expected, the GM CBF values were higher than the WM CBF values. We also observed variations in these age- and sex-related differences across different brain regions, as expected. Further, various cortical GM regions investigated exhibited a statistically significant (p < 0.05), or close to significant (p < 0.1), decline in CBF with age (Table 1), in agreement with recent studies (4, 5). Interestingly, the WM regions exhibited increasing trends of CBF with age; this association was significant in all brain regions studied except the temporal lobes (Table 1). We provisionally attribute this increase of CBF with age in WM to increasing oligodendrocyte metabolic demand for maintenance of myelin homeostasis (6, 7).Sex differences in CBF were statistically significant in most GM and WM regions studied (Table 1). Our results indicate that women exhibit, overall, higher mean CBF values than males (Fig. 4), in agreement with recent work (4). This may be accounted for by differential endocrine regulation of CBF between the sexes (4). In addition, recent studies have also indicated greater myelin content in women as compared to men (6, 7). Thus, greater CBF in women could reflect increased oligodendrocyte metabolic demand for myelin synthesis.
CONCLUSIONS
Our results provide evidence for statistically significant associations between age and sex, and CBF, in a large sample of well-characterized cognitively unimpaired adults. The GM regions exhibit overall statistically significant decreasing CBF with age while WM exhibited increasing trends. Finally, women showed higher CBF values than men in all brain structures investigated.Acknowledgements
This work was supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health.References
1. Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, et al. Recommended Implementation of Arterial Spin Labeled Perfusion MRI for Clinical Applications: A consensus of the ISMRM Perfusion Study Group and the European Consortium for ASL in Dementia. Magnetic resonance in medicine. 2015;73(1):102-16.
2. Bouhrara M, Lee DY, Rejimon AC, Bergeron CM, Spencer RG. Spatially adaptive unsupervised multispectral nonlocal filtering for improved cerebral blood flow mapping using arterial spin labeling magnetic resonance imaging. Journal of Neuroscience Methods. 2018.
3. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62(2):782-90.
4. Liu W, Lou X, Ma L. Use of 3D pseudo-continuous arterial spin labeling to characterize sex and age differences in cerebral blood flow. Neuroradiology. 2016;58(9):943-8.
5. Chen JJ, Rosas HD, Salat DH. Age-associated reductions in cerebral blood flow are independent from regional atrophy. NeuroImage. 2011;55(2):468-78.
6. Bouhrara M, Rejimon AC, Cortina LE, Khattar N, Bergeron CM, Ferrucci L, et al. Adult brain aging investigated using BMC-mcDESPOT based myelin water fraction imaging. Neurobiology of Aging. 2019.
7. Arshad M, Stanley JA, Raz N. Adult age differences in subcortical myelin content are consistent with protracted myelination and unrelated to diffusion tensor imaging indices. Neuroimage. 2016;143:26-39.
Figures