0187
Increased Blood-Brain Interface Water Permeability in the Ageing Brain detected using non-invasive Multiple Echo Time ASL MRI1Centre for Advanced Biomedical Imaging, UCL, London, United Kingdom, 2Neuroradiological Academic Unit, UCL Queen Square Institute of Neurology, UCL, London, United Kingdom, 3Dementia Research Centre, UCL Queen Square Institute of Neurology, UCL, London, United Kingdom, 4Wellcome Centre for Humans Neuroimaging, UCL Queen Square Institute of Neurology, UCL, London, United Kingdom
Synopsis
Multi-TE ASL technique detects a significant increase (32%) in blood-brain interface (BBI) permeability in the ageing brain. The change in BBI water permeability is associated with a marked increase (1.9 ± 0.4 fold) in expression of PDGFRβ, an index of pericyte coverage, and changes to aquaporin water channels and their anchoring proteins in the ageing brain. This technique is a promising non-invasive tool to measure age-related changes to the BBI, that may play a mechanistic role in the pathogenesis of neurodegenerative conditions.
Introduction
There is increasing evidence to suggest that changes to the integrity of the blood-brain interface (BBI) is an early event in neurodegenerative conditions, such as Alzheimer’s Disease (AD) [1-3]. BBI dysfunction is associated with inflammation, loss of tight junctions, pericyte coverage and changes to astrocytic aquaporin-4 (AQP4) water channels, potentially causing an increase in BBI permeability [4]. BBI permeability is often assessed using DCE-MRI, which uses relatively large contrast agents, that may only detect gross damage to the BBI [1]. Given that ageing is the biggest risk factor for AD [5], aged mice may represent a model of early neurodegenerative processes in age-related cognitive decline [6]. Non-invasive multiple echo time (multi-TE) ASL uses the endogenous vascular water as a tracer, to measure the rate of vascular water moving into the extravascular brain tissue as an index of BBI permeability to water. We hypothesize that BBI permeability to water may be increased in the aged mouse, due to dysfunction to the components of the BBI that can occur during the ageing process. Detecting changes in BBI permeability to water in the aged mouse brain could help to understand early neurodegenerative processes that may be a preclinical precursor of AD.Methods
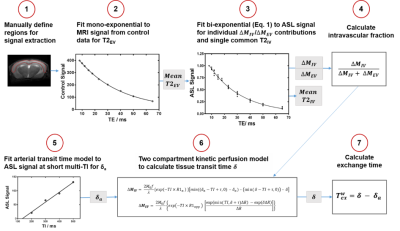
Images were acquired using a 9.4T Bruker imaging system (BioSpec) in two groups of C57Bl/6JRj mice, aged mice at 27±1 month old (n = 8) and adult mice at 7±1 month old (n = 9). Imaging parameters were: inflow times (TI) = 800 ms and 1500 ms; echo times (TE) = 8, 10, 12, 15, 18, 23, 30, 40, 50, 65 ms; slice thickness = 2 mm; FOV = 25x25 mm; matrix size = 64x64; repetitions = 10. All mice were anesthetised with 2% isoflurane in a mixture of 1.0L/min medical air, and adjusted to maintain respiration at ~100bpm. Multi-TE ASL data was processed with Matlab R2018 (Mathworks), using protocol shown in Figure 1, which uses a bi-exponential (intravascular (IV) and extravascular (EV)) model for ASL signal, ΔM = ΔMIV exp((TE)/(T2IV)) + ΔMEV exp((TE)/(T2EV)) (Eq.1) to calculate the mean cortical exchange time (Texw) as a surrogate index of the BBI water permeability [7]. CBF was determined using the general kinetic model.For quantification of BBI molecular components, mRNA expression of aquaporin-4 water channels (Aqp4), α-syntrophin protein (SNTA1) for anchoring of AQP4 to BBI, platelet-derived growth factor receptor-β (PDGFRβ), as an index of pericytes coverage and the tight junction proteins, occludin (Occl) and claudin-5 (Cld5) were measured. The cortical regions of the same aged mice (n = 7) and adult mice (n = 7) were dissected, total RNA was extracted and converted to cDNA using RNeasy® Plus Microkit/ QuantiTect® Reverse Transcription Kit (Qiagen). TaqMan® Gene Expression assays were used for each BBI component, normalising to housekeeper genes (ACTB and GAPDH). Quantification of mRNA expression level, for each BBI component was assessed by the 2–∆∆Ct method [8].
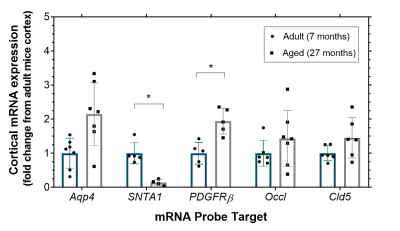
Methods
The mean cortical exchange time was significantly (32%) lower in the aged mice (335 ± 70 ms) relative to the adult mice (440 ± 75 ms) (p = 0.017), Figure 2. There were no marked differences in CBF in aged mice (188 ± 61 ml/100g/min) compared to the adult mice (191 ± 71 ml/100g/min), p = 0.935. The changes to the mRNA expression of the molecular components of the BBI of the aged mice relative to the adult mice are presented in Figure 3. We report an increase, though non-significant, to the mRNA expression of AQP4 in the aged mice (2.1 ± 0.9 fold), p = 0.083. There was a significant decrease in SNTA1 mRNA expression in the aged mice (0.14 ± 0.09 fold), p = 0.012. There is a marked increase in mRNA expression of PDGFRβ (1.9 ± 0.4 fold) in the aged mice, p = 0.015. mRNA expression of tight junction proteins occludin (1.4 ± 0.8 fold) and claudin-5 (1.5 ± 0.6 fold) appear to be relatively maintained in ageing brain, p > 0.999 and p = 0.645 respectively.Discussion
Multi-TE ASL detected a significantly lower exchange time as an index of increased BBI water permeability in the cortical region of the ageing mouse brain. This represents the first measurement of changes to BBI permeability to water in the ageing brain. The increase in BBI water permeability may be due to the increase in the expression of pericytes marker PDGFRβ. Recent studies have reported an increase in soluble PDGFRβ to reflect pericyte injury at the BBI and correlate with the extent of cognitive decline in early MCI patients [3]. The increase in water permeability in the ageing brain may also be associated with the mean increase in AQP4 water channel (although conversely this concurs with significant decrease in protein, SNTA1 that anchors the AQP4 to the BBI). The changes to AQP4 and SNTA1 suggest dysfunction of AQP4 channels, which has previously been associated with the severity cognitive decline in AD [9].Conclusion
Non-invasive multi-TE ASL can detect an increase in BBI permeability to water in the ageing mouse brain. This technique may be used to better understand the changes occurring at the BBI that may play a key mechanistic role in the pathogenesis of neurodegenerative conditions.Acknowledgements
This work is supported by the Medical Research Council (MR/K501268/1), the EPSRC-funded UCL Centre for Doctoral Training in Medical Imaging(EP/L016478/1) and the UCL Leonard Wolfson Experimental Neurology Centre (PR/YLR/18575), together with the Wellcome Trust and Royal Society.References
[1] Montagne, A., S. R. Barnes, M. D. Sweeney, M. R. Halliday, A. P. Sagare, Z. Zhao, A. W. Toga, R. E. Jacobs, C. Y. Liu, L. Amezcua, M. G. Harrington, H. C. Chui, M. Law and B. V. Zlokovic (2015). "Blood-brain barrier breakdown in the aging human hippocampus." Neuron 85(2): 296-302.
[2] van de Haar, H. J., S. Burgmans, J. F. Jansen, M. J. van Osch, M. A. van Buchem, M. Muller, P. A. Hofman, F. R. Verhey and W. H. Backes (2016). "Blood-Brain Barrier Leakage in Patients with Early Alzheimer Disease." Radiology 281(2): 527-535.
[3] Nation, D. A., M. D. Sweeney, A. Montagne, A. P. Sagare, L. M. D’Orazio, M. Pachicano, F. Sepehrband, A. R. Nelson, D. P. Buennagel, M. G. Harrington, T. L. S. Benzinger, A. M. Fagan, J. M. Ringman, L. S. Schneider, J. C. Morris, H. C. Chui, M. Law, A. W. Toga and B. V. Zlokovic (2019). "Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction." Nature Medicine 25(2): 270-276.
[4] Erdő, F., L. Denes and E. de Lange (2017). "Age-associated physiological and pathological changes at the blood-brain barrier: A review." Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 37(1): 4-24.
[5] Farrall, A. J. and J. M. Wardlaw (2009). "Blood-brain barrier: ageing and microvascular disease--systematic review and meta-analysis." Neurobiol Aging 30(3): 337-352.
[6] Ahlemeyer, B., S. Halupczok, E. Rodenberg-Frank, K. P. Valerius and E. Baumgart-Vogt (2018). "Endogenous Murine Amyloid-beta Peptide Assembles into Aggregates in the Aged C57BL/6J Mouse Suggesting These Animals as a Model to Study Pathogenesis of Amyloid-beta Plaque Formation." J Alzheimers Dis 61(4): 1425-1450.
[7] Ohene, Y., I. F. Harrison, P. Nahavandi, O. Ismail, E. V. Bird, O. P. Ottersen, E. A. Nagelhus, D. L. Thomas, M. F. Lythgoe and J. A. Wells (2019). "Non-invasive MRI of brain clearance pathways using multiple echo time arterial spin labelling: an aquaporin-4 study." NeuroImage 188: 515-523.
[8] Livak, K. J. and T. D. Schmittgen (2001). "Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method." Methods 25(4): 402-408.
[9] Zeppenfeld, D. M., M. Simon, J. D. Haswell, D. D’Abreo, C. Murchison, J. F. Quinn, M. R. Grafe, R. L. Woltjer, J. Kaye and J. J. Iliff (2017). "Association of Perivascular Localization of Aquaporin-4 With Cognition and Alzheimer Disease in Aging Brains." JAMA Neurology 74(1): 91
Figures