0186
Perivascular space imaging across the lifespan1Mark and Mary Stevens Institute for Neuroimaging and Informatics, University of Southern California, Los Angeles, CA, United States
Synopsis
The perivascular space (PVS) is a major component of the glymphatic system and it promotes functional brain clearance. PVS enlargement has been observed in neurological disorders and is considered a biomarker for vascular pathology, however its role in normative development is not well understood. Using a novel technique to segment PVS, we sought to quantify age-related changes in PVS across the lifespan in a large cross-sectional cohort of cognitively normal individuals. We found age was significantly and positively associated with PVS throughout the brain and these results provide a first step towards understanding the typical evolution of brain clearance mechanisms.
Introduction
The glymphatic system responsible for brain clearance promotes the efficient elimination of waste and excess fluid from the central nervous system [1] and proper functionality of these processes is critical for tissue homeostasis [2]. The perivascular space (PVS) is a major component of the glymphatic system and consists of tubular, interstitial fluid-filled cavities that surround small penetrating vessels and provide a low-resistance pathway for periarterial cerebrospinal fluid (CSF) flow to facilitate the drainage of interstitial solutes [1], [3]. PVS enlargement has been observed in normal aging [4] and is implicated in a variety of neurological disorders characterized by atypical waste clearance including traumatic brain injury [5], stroke [6] and Alzheimer’s Disease [7]. While PVS dilation may represent an important biomarker for vascular pathology in the brain, its role in normative development and aging across the lifespan is poorly understood. Our colleagues have recently developed a novel neuroimaging processing workflow to automatically identify and quantify PVS in the brain in vivo using multi-modal structural neuroimaging contrasts [8]. The goal of this study was to use this approach to characterize regional PVS changes in white matter across the lifespan. Here, we quantify and map age-related changes in PVS content in a large cross-sectional cohort (~2000) of typically developing and cognitively normal children, adults and the elderly. These results provide a normative reference for the localization and extent of PVS distributions from which pathological alterations can be compared.Methods
Neuroimaging data from 2037 cross-sectional cognitively normal subjects between 8 and 100 years of age (34.07±20.8 years; 1105 F) were obtained from the Lifespan Human Connectome Project (HCP) cohorts: HCP Development, HCP Adults, and HCP-Aging. High resolution T1-weighted (T1w) MPRAGE scans (voxel size: .7 mm isotropic; FOV: 224x224 mm; TI: 1000 ms; TR/TE: 2400/2.14 ms) and T2-weighted (T2w-SPC) scans (voxel size: .7 mm isotropic; FOV: 224x224 mm; TR/TE: 3200/565 ms) were used for the present study. Slight variations in the acquisition parameters were made for the HCP-Aging and HCP Development cohorts to accommodate the unique challenges of working with young and elderly populations [9]. PVS segmentation was performed using the methods described in [8]. Briefly, structural MRI data was preprocessed using the HCP pipeline [10], scans were co-registered to ensure correspondence, and adaptive non-local mean filtering was applied [11]. To explore regional age-related changes to PVS in white matter, T1w images were parcellated into distinct white matter regions using Freesurfer (http://surfer.nmr.mgh.harvard.edu/). In order to reliably extract PVS, an Enhanced PVS Contrast (EPC) image was obtained by dividing the filtered structural scans (T1w/T2w). Vesselness maps were obtained through Frangi filtration [12] and then PVS was automatically segmented and quantified using optimal thresholding of the maps. The PVS volume fraction for each white matter region was calculated by dividing the PVS volume by the white matter volume. General linear models were then applied to regional PVS volume fractions to calculate the main effect of age while controlling for demographic variables, such as sex. To ensure the observed changes to PVS were due to age, models were also tested in the 3 cohorts separately.Results
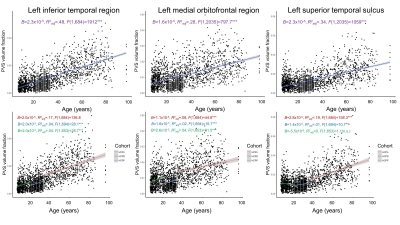
Age was significantly and positively associated with PVS in all white matter regions tested (Figure 1). The most significant associations between PVS and age were observed in the left and right inferior temporal white matter (Right: Beta=2.23x10-5, t(2034)=44.22, p<.001; Left: Beta=2.32x10-5, t(2034)=44.23, p<.001), where age and sex explain a significant amount of variance in PVS content (Right: F(2,2034)=980.87, p<.001, R2adjusted=.49; Left: F(2,2034)=982.51, p<.001, R2adjusted=.49). These significant age associations remained when analyses were stratified by cohort, however age was not significantly associated with PVS content in regions such as the left superior temporal sulcus when the HCP Development cohort was isolated (Figure 1). The analysis shows that sex was significantly associated with PVS content in the majority of tracts, with males having increased PVS volume fractions compared to females globally; however sex did not significantly predict PVS content in bilateral transverse temporal region (p=.14), superior temporal sulcus (p=.08), and pericalcarine regions (p=.1). Across all regions, PVS volume fraction variability increased with age.Discussion
Our results show that the fraction of tissue occupied by PVS increases significantly with age across the lifespan in cognitively normal individuals. Aging is associated with dysfunction of the clearance system [4], which may be attributed to a number of cellular changes observed in elderly subjects. For example, arterial pulsatility decreases with age, which can alter CSF influx and negatively impact glymphatic function [13]. There is also evidence that the blood-brain barrier is disrupted during aging, which can further contribute to the pathogenesis of PVS dilation or development of cerebral small vessel disease [14]. Overall, our results show that while PVS enlargement is observed across development, these age-related changes are driven in large part by the aging cohort.Conclusion
PVS imaging methods provide invaluable tools to probe vascular and glymphatic processes that may be predictive of vascular pathology. The present study demonstrates that PVS enlargement is significantly associated with age across the lifespan and provides an important reference point from which to understand neurological disorders characterized by abnormal brain clearance.Acknowledgements
This work was supported by NIH grants: 2P41EB015922-21, 1P01AG052350-01 and USC ADRC 5P50AG005142. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.References
[1] J. J. Iliff et al., “A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β,” Sci. Transl. Med., vol. 4, no. 147, 2012.
[2] N. A. Jessen, A. S. F. Munk, I. Lundgaard, and M. Nedergaard, “The Glymphatic System – A Beginner’s Guide,” Neurochem Res, vol. 40, no. 12, pp. 2583–2599, 2015.
[3] M. Marín-Padilla and D. S. Knopman, “Developmental aspects of the intracerebral microvasculature and perivascular spaces: Insights into brain response to late-life diseases,” J. Neuropathol. Exp. Neurol., vol. 70, no. 12, pp. 1060–1069, 2011.
[4] B. T. Kress et al., “Impairment of paravascular clearance pathways in the aging brain,” Ann. Neurol., vol. 76, no. 6, pp. 845–861, 2014.
[5] R. A. Opel et al., “Effects of traumatic brain injury on sleep and enlarged perivascular spaces,” J. Cereb. Blood Flow Metab., 2018.
[6] A. Charidimou et al., “Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: A multicentre MRI cohort study,” J. Neurol. Neurosurg. Psychiatry, vol. 84, no. 6, pp. 624–629, 2013.
[7] G. Banerjee et al., “MRI-visible perivascular space location is associated with Alzheimer’s disease independently of amyloid burden.,” Brain, vol. 140, no. 4, pp. 1107–1116, Apr. 2017.
[8] F. Sepehrband et al., “Image processing approaches to enhance perivascular space visibility and quantification using MRI,” Sci. Rep., vol. 9, p. 12351, Aug. 2019.
[9] M. Harms et al., “Extending the Human Connectome Project across ages: Imaging protocols for the Lifespan Development and Aging projects,” Neuroimage, vol. 183, pp. 972–984, 2018.
[10] M. F. Glasser et al., “The minimal preprocessing pipelines for the Human Connectome Project,” Neuroimage, vol. 80, pp. 105–124, 2013.
[11] J. V Manjón, P. Coupé, L. Martí‐Bonmatí, D. L. Collins, and M. Robles, “Adaptive non‐local means denoising of MR images with spatially varying noise levels,” J. Magn. Reson. Imaging, vol. 31, no. 1, pp. 192–203, 2010.
[12] A. F. Frangi, W. J. Niessen, K. L. Vincken, and M. A. Viergever, “Multiscale vessel enhancement filtering,” in International Conference on Medical Image Computing and Computer-Assisted Intervention, 1998, pp. 130–137.
[13] J. J. Iliff et al., “Cerebral arterial pulsation drives paravascular CSF–interstitial fluid exchange in the murine brain,” J. Neurosci., vol. 33, no. 46, pp. 18190–18199, 2013.
[14] Y. Li et al., “The relationship between blood–brain barrier permeability and enlarged perivascular spaces: A cross-sectional study,” Clin. Interv. Aging, vol. 14, pp. 871–878, 2019.
Figures