0178
In-vivo Electromagnetic Field Mapping for Transcranial Electrical Stimulation (tES) using Deep Learning1School of Biological and Health Systems Engineering, Arizona State University, Tempe, AZ, United States
Synopsis
Magnetic flux densities induced by tES currents can be measured from MR phase and used to reconstruct current density, electric field and conductivity tensor distributions, via diffusion tensor magnetic resonance electrical impedance tomography (DT-MREIT). Determination of tES electric field distributions from DT-MREIT conductivities is challenging, because DT-MREIT requires data from two independent current administrations, increasing acquisition time. We demonstrate a deep-learning model for DT-MREIT reconstruction, showing that conductivity tensors and electric fields can be measured in human subjects in-vivo using a single current administration. This strategy can be used to directly monitor tES electric fields and verify treatment precision.
INTRODUCTION:
Transcranial electrical stimulation (tES) is a non-invasive neuromodulation technique indicated to treat depression and chronic pain1. In tES, low amplitude current is delivered to the brain through surface electrodes2. The best contemporary estimates of electric fields delivered by tES are estimated from computational models of the head3. However, these models rely on literature values for tissue conductivities and do not include electrode contact impedances. Therefore, this approach cannot correctly predict subject-specific fields. DT-MREIT4 has recently been introduced to produce electrical conductivity tensor images by imaging of a scale factor ($$$\eta$$$) relating MRI diffusion and conductivity tensors. Stable reconstruction of $$$\mathbf{C}$$$ via DT-MREIT requires two independent current administrations. Here, we use data from an in-vivo human experiment to demonstrate that it is possible to reconstruct $$$\mathbf{C}$$$ using a single experimental current administration.METHODS:
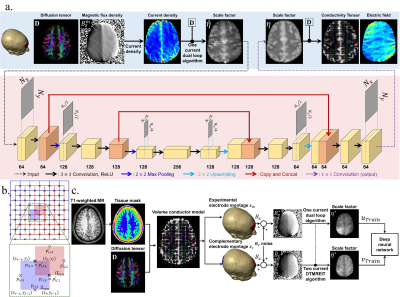
Experiment: Experimental protocols were approved by the Arizona State University Institutional Review Board. Data obtained from a 58-year old volunteer male human subject was used to demonstrate method performance. Data were measured using a 32-channel RF head coil in a 3.0T Phillips scanner (Phillips, Ingenia, Netherlands) located at the Barrow Neurological Institute (Phoenix, Arizona, USA). A transcranial electrical stimulator (DC-STIMULATOR MR, neuroConn, Ilmenau, Germany) was used to deliver 1.5 mA currents using an Fpz-Oz electrode montage. Sets of $$$B_z^m$$$ data were measured on three axial slices using a multi-echo gradient-echo pulse sequence and an image matrix size of 128$$$\times$$$128. Other imaging parameters were, TR/TE/ES=50/7/3 ms, FOV= 224$$$\times$$$ mm2, slice thickness, 5 mm, and number of echoes, NE = 10. Individual echo images were then combined to improve the SNR of the acquired $$$B_z^m$$$ signal5. Prior to echo combination, stray magnetic field corrections were applied to each echo image6. Figure 1 (b) and (d) show acquired MR magnitude and echo-combined $$$B_z^{opt}$$$ images respectively for the center slice. Diffusion-weighted images of the three MREIT slices were also obtained, using an SS-SE-EPI imaging sequence, with $$$b$$$ of 1000 sec/mm2 and fifteen diffusion directions. The FA map is shown in Fig. 1(e). High-resolution T1-weighted images were also collected to construct computational models of the head and lead wires (Fig 1a).Scale-factor reconstruction: We applied the KVL in a rectangular region $$$\Omega_{ij}$$$, $$$\Omega_{ij}^{'} \in \Omega_t$$$ where, $$$\Omega_t$$$ is the DT-MREIT slice at $$$z=t$$$ (Fig. 2b). A dual-loop relationship relating the water diffusion tensor $$$\mathbf{D}$$$, estimated regional current density7 $$$\mathbf{J}^{P}$$$ from experimentally obtained $$$B_z^{opt}$$$ data and the target $$$\hat{\eta}$$$ was formulated as
$$\begin{cases}\frac{\left(\mathbf{D}^{-1}\mathbf{J}^P\right)_x\left(p_{ij,1}\right)}{\hat{\eta}(x_i,y_{j-1})}+\frac{\left(\mathbf{D}^{-1}\mathbf{J}^P\right)_y\left(p_{ij,2}\right)}{\hat{\eta}(x_i,y_j)}-\frac{\left(\mathbf{D}^{-1}\mathbf{J}^P\right)_x\left(p_{ij,3}\right)}{\hat{\eta}(x_i,y_j)}-\frac{\left(\mathbf{D}^{-1}\mathbf{J}^P\right)_y\left(p_{ij,4}\right)}{\hat{\eta}(x_{i-1},y_j)}=0&\mbox{in}~\Omega_{ij}\\\frac{\left(\mathbf{D}^{-1}\mathbf{J}^P\right)_x\left(p^{'}_{ij,1}\right)}{\hat{\eta}(x_i,y_j)}+\frac{\left(\mathbf{D}^{-1}\mathbf{J}^P\right)_y\left(p^{'}_{ij,2}\right)}{\hat{\eta}(x_{i+1},y_j)}-\frac{\left(\mathbf{D}^{-1}\mathbf{J}^P\right)_x\left(p^{'}_{ij,3}\right)}{\hat{\eta}(x_i,y_{j+1})}-\frac{\left(\mathbf{D}^{-1}\mathbf{J}^P\right)_y\left(p^{'}_{ij,4}\right)}{\hat{\eta}(x_i,y_j)}=0&\mbox{in}~\Omega^{'}_{ij}\end{cases} (1) $$
Scale factor values at the boundary were assumed, and an overdetermined system was built comprising $$$2\left(N-2\right)^2$$$ equations, which was then solved for $$$\left(N-2\right)^2$$$ internal nodes.
Dual-loop $$$\hat{\eta}$$$ reconstructions were affected by streaking artefacts due to noise propagation along equipotential lines (Fig. 3b left). To overcome this, a regressor model of non-linear artefact functions was built using a deep neural network8,9 (Fig. 2a). Training datasets were obtained from numerical simulations only. Scale factor values were assumed piecewise constant in each tissue (GM, WM, CSF), and the DT-MREIT forward problem4 was been solved for 1000 numerical models (WM: 0.2-0.775, GM: 0.2-0.775, CSF: 0.4-0.8 S•sec/mm3 with 0.1 S•sec/mm3 linear step) using an FPz-Oz electrode montage. Noise of 0.21 nT was added to each simulated $$$B_z^{*}$$$ image10. Artefact-affected $$$\eta^{*}$$$ images were then reconstructed using (1). Data from a complementary electrode montage (T7-T8) was simulated and used to reconstruct artifact-free images of $$$\tilde{\eta}^{*}$$$ using the two-current injection DT-MREIT method4 (Fig. 2c). These two training datasets were used to train a U-net architecture (Fig. 2a) implemented in Keras library in Python. By minimizing $$$l^2$$$ loss function, 1,327,744 parameters were trained using the RMSProp optimizer with 32-mini-batch size and 1000 epochs.
Electric field reconstruction: The conductivity tensor $$$\mathbf{C} = \eta \mathbf{D}$$$ was calculated using scale-factors from the trained network (Fig. 2a). Electric field distributions were then estimated using $$$\mathbf{E} = \mathbf{C}^{-1} \mathbf{J}^P$$$.
Verification: Reconstruction performance was verified using relative $$$L^2$$$-differences from standard scale-factor images obtained using the DT-MREIT algorithm3 and another set of $$$B_z^m$$$ data measured using a T7-T8 electrode pair.
RESULTS:
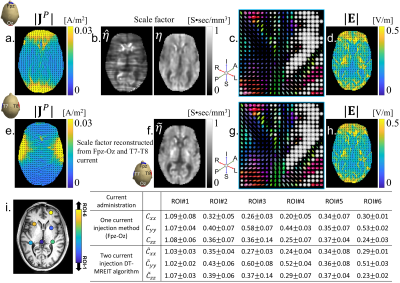
Figure 3(a) shows estimated projected current density caused by current flow through the Fpz-Oz electrode montage. Reconstructed scale factor images from (1) are in Fig. 3(b-left). As expected, the reconstructed scale factor image from one current injection was corrupted by streaking artefacts. Results obtained after correction using the deep neural network (Fig. 2a) are in Fig. 3(b-right). The neural-network corrected scale factor image was multiplied with measured diffusion tensor data to form conductivity tensors4 (Fig. 3(c)) shows the reconstructed tensor within the ROI (Fig. 1b). Reconstructed electric fields are shown in Fig. 3d and compared with those from the two-current administration method4 (Fig. 3e-i). Relative $$$L^2$$$-differences between single-current injection and standard-reconstructed $$$\eta$$$ and derived electric fields were found to be 0.15 and 0.20 respectively.DISCUSSION:
We have demonstrated that it is possible to reconstruct the conductivity tensor with reasonable accuracy using single current data. One of the drawbacks of this implementation is the limited number of training datasets. We plan to use this method to reconstruct the electromagnetic field for F3-F4 electrode montages using larger numbers of training datasets.CONCLUSIONS:
Results of in-vivo human experiments demonstrate that stable conductivity tensors can be reconstructed using only one current injection.Acknowledgements
This work was supported by award RF1MH114290 to RJS.References
1. Fregni F, Boggio P S and Lima M C. et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006; 122(1-2): 197-209.
2. Nitche M A and Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000; 527(3), 633-39.
3. Datta A, Truong D and Minhas P et al. Inter-individual variation during transcranial direct current stimulation and normalization of dose using MRI-derived computational models. Front. Psych. 2012, 3:1-8.
4. Kwon O I, Jeong W C and Sajib S Z K et al. Anisotropic conductivity tensor imaging in MREIT using directional diffusion rate of water molecules. Phys. Med. Biol. 2014; 59 (12): 2955-74.
5. Kim M N, Ha T Y, Woo E J et al. Improved conductivity reconstruction from multi-echo MREIT utilizing weighted voxel-specific signal-to-noise ratios. Phys. Med. Biol. 2012; 57 (11):3643-59.
6. Sajib S Z K, Chauhan M and Banan G et al. Compensation of lead-wire magnetic field contributions in MREIT experiment using image segmentation: a phantom study. In Proc. Int. Soc. Magn. Reson. Med. 2019; 5049.
7. Sajib S Z K, Kim H J, Kwon O I et al. Regional absolute conductivity reconstruction using projected current density in MREIT. Phys. Med. Biol. 2012; 57 (18):5841-59.
8. Ronneberger O, Fischer P and Brox T. U-net: convolutional networks for biomedical image segmentation arXiv:1505.04597, 2015.
9. Hyun C M, Kim H P and Lee S M et al. Deep learning for undersampled MRI reconstruction. Phys. Med. Biol. 2018; 63(13): 15 pp.
10. Sadleir R J, Grant S, Zhang S U et al. Noise analysis in MREIT at 3 and 11 Tesla field strength. Physiol. Meas. 2005; 27(5):875-84.
Figures