0177
Dynamic Susceptibility Contrast with Undersampled Golden-Angle Radial Imaging in the Rodent Spinal Cord1Biophysics, Medical College of Wisconsin, Milwaukee, WI, United States, 2Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
Dynamic susceptibility contrast (DSC) to monitor spinal cord perfusion and hemodynamics has the potential to inform the clinical care of spinal cord injury and other disorders. Acquisition of high spatial and temporal resolution images of the rodent spinal cord for DSC perfusion measurements was achieved using a golden-angle radial gradient-echo acquisition combined with iGRASP iterative undersampled reconstruction.
Introduction
Dynamic susceptibility contrast (DSC) imaging uses a bolus injection of an exogenous gadolinium-based contrast agent and rapid imaging within the first passage of the agent through the vasculature to derive perfusion estimates. This technique is established in the brain but there is limited application to the spinal cord despite its potential application to monitor spinal cord blood flow in spinal cord injury or other disorders1,2. MRI of the spinal cord is more challenging than brain MRI due to the small size of the cord, its propensity for motion, and distortions due to the surrounding vertebral column. Rapid imaging is necessary with temporal resolutions in the human of 1 second or less to accurately characterize the bolus through the tissue. Higher temporal resolution is necessary in small animals. We applied golden-angle radial acquisition combined with an iterative undersampling reconstruction technique to achieve DSC images with a temporal resolution of 0.48 s.Methods
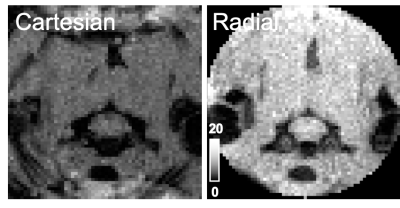
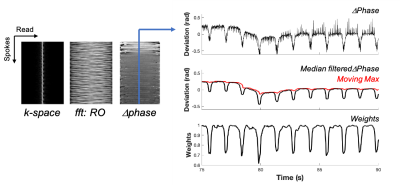
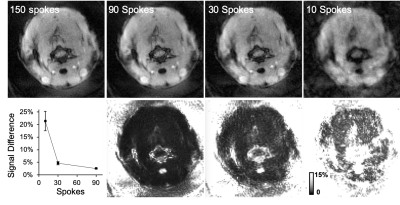
Adult female Sprague-Dawley rats were used in this study. MRI was performed on a 9.4T Bruker Biospec (Paravision 6.0.1) and that animal was positioned in a head holder inside a 38 diameter Litz coil (Doty Scientific, Inc). Cartesian gradient echo imaging was initially compared with a golden-angle radial acquisition using a modified ultra-short TE (UTE) pulse sequence with axial images (TR/TE = 10/3.5 ms, resolution = 0.375 x 0.375 mm). Subsequently, three slice, axial DSC images were collected with the golden-angle radial scheme (TR/TE = 16/3.5 ms, resolution = 0.375 x 0.375 mm) with 150 spokes (fully sampled) and a temporal resolution of 2.4 s. A bolus of gadodiamide (0.1mmol/kg) was injected through a tail vein catheter after 80 s of precontrast scans. Image reconstruction used a nonuniform fast Fourier transform (NUFFT) on the Bruker system and was compared to an off-line, iterative undersampling reconstruction technique, iGRASP3. Data was undersampled using 12 iGRASP iterations and either 90, 30 or 10 spokes. The average signal difference was calculated from 23 timelocked images of fully sampled and undersampled datasets across the precontrast baseline images. The respiratory trace was derived from the phase of the center point for each spoke and used as a weighting parameter in the reconstruction. Evaluation determined undersampling with 30 spokes provided sufficient image quality with a temporal resolution of 0.48 s and this reconstruction was used for subsequent perfusion analysis. The dynamic transverse relaxation rate constant (R2*) within a manually drawn spinal cord ROI was calculated: ∆R2*t = -(1/TE)*ln(St/S0) where S0 is the precontrast signal average. Relative perfusion estimates of time-to-peak (rTTP), the time from bolus injection to peak signal intensity, from the SCI epicenter at C5 cervical level are reported.Results
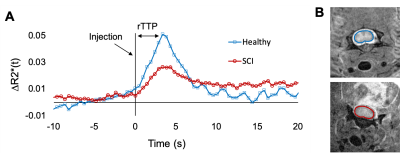
Compared to Cartesian sampling, the golden radial acquisition had higher temporal signal to noise (tSNR) of 7.1 and 14.5, respectively. Average signal change compared to fully sampled data in a manually drawn spinal cord ROI was 2.5 ± 0.2, 4.7 ± 0.6 and 21.4 ± 3.9 % for undersampling rates of 90, 30, and 10 spokes respectively. iGRASP undersampling factors in the healthy rat determined image quality was maintained at an undersampling rate of 30 spokes, which provided a 5x increase in temporal resolution (0.48 s). Image quality notably deteriorated with only 10 spokes. The mean R2* was plotted for both a healthy and a SCI rat and derived estimates of rTTP were 3.36 and 3.84 s, respectively. Increased post bolus signal (∆R2*) in the injured animal is suggestive of contrast agent leakage which was not accounted for in the current modelling.Discussion
Golden-angle radial acquisition combined with iGRASP iterative undersampled reconstruction achieved quality DSC images at high temporal resolution. We demonstrated feasibility of this technique in an animal model. The phase derived respiratory signal provides weighting information for reconstruction robust to respiratory motion. Further experiments are necessary to address leakage effects which are known to alter perfusion measurements because of both T1 and T2* effects and is expected in the traumatically injured cord4,5. Relative time to peak was increased in the SCI animal and further optimization including leakage correction is required to obtain perfusion parameters such as mean transit time and spinal cord blood flow.Conclusion
We have reported implementation of DSC imaging in the rat cervical spinal cord that is relatively robust to respiratory effects that typically confound dynamic imaging. Continued development of this perfusion technique can provide MRI methods to monitor spinal cord blood flow in injury or compression.Acknowledgements
We thank Natasha Wilkins and Matt Runquist for experimental assistance. This study was supported by funding from the National Institutes of Neurological Disorders and Stroke (R01NS109090).References
1. Ellingson, B. M., Woodworth, D. C., Leu, K., Salamon, N. & Holly, L. T. Spinal cord perfusion MR imaging implicates both ischemia and hypoxia in the pathogenesis of cervical spondylosis. World Neurosurg. (2019). doi:10.1016/j.wneu.2019.04.253
2. Liu, X., Germin, B. I. & Ekholm, S. A case of cervical spinal cord glioblastoma diagnosed with MR diffusion tensor and perfusion imaging. J. Neuroimaging 21, 292–296 (2011).
3. Feng, L. et al. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn. Reson. Med. 72, 707–717 (2014).
4. Stokes, A. M., Semmineh, N. & Quarles, C. C. Validation of a T1 and T2* leakage correction method based on multiecho dynamic susceptibility contrast MRI using MION as a reference standard. Magn. Reson. Med. 76, 613–625 (2016).
5. Paulson, E. S. & Schmainda, K. M. Comparison of Dynamic Susceptibility-weighted Contrast-enhanced MR Methods: Recommendations for Measuring Relative Cerebral Blood Volume in Brain Tumors. Radiology 249, 601–613 (2008).
Figures