0174
Quantifying the Transchelation of Gd3+ Ions from Linear and Macrocyclic GBCA to Glycosaminoglycans using MR Relaxometry1Leibniz-Forschungsinstitut fuer Molekulare Pharmakologie (FMP), Berlin, Germany, 2BIOQIC, Charité Berlin, Berlin, Germany, 3Department of Radiology, Charité Berlin, Berlin, Germany
Synopsis
In this study, we quantified the dissociation of GBCAs at different ZnCl2 concentrations and the subsequent chelation of Gd3+ to glycosaminoglycans (GAGs) like heparin. We showed that the relaxivity of the resulting Gd-GAG complexes is about seven times higher compared to that of GBCAs. Under physiological conditions, we further showed that ~20% of the Gd3+-ions transchelated from linear GBCAs to heparin and that these are accountable for more than 50% of the observed relaxation rate. Therefore, Gd-GAG complexes should be considered as the Gd-containing macromolecular substances with high relaxivity that are needed to explain the observed long-term enhancements in vivo.
Introduction
Several studies reported that gadolinium ions (Gd3+) can be released from their chelators in the presence of competing ions and then remain in various tissues in the body[1-3]. This was observed as hyperintensities in T1-weighted images of patients that received several GBCA administrations[4-6]. However, the exact mechanism of Gd deposition (e.g., the molecular form of the retained Gd-containing species) is not fully understood[2,3]. In this study, we quantify the release of Gd3+ ions from GBCAs and the subsequent chelation to glycosaminoglycans (GAGs), which could be a potential explanation for the observed long-term enhancements in vivo.Methods
All MR measurements were performed on a 9.4T MRI system (Bruker, Ettlingen, Germany) at T = 25 °C. T1 measurements were performed using a saturation recovery MRI sequence and all quantitative values were obtained by fitting ROI-averaged signals. Heparin was used as model GAG, Gd-DTPA (Magnevist®, Bayer AG, Germany) and Gd-DTPA-BMEA (Optimark®, Covidien, Ireland) as representative linear GBCAs, and Gd-DOTA (Dotarem®, Guerbet, France) and Gd-DO3A-butrol (Gadovist®, Bayer AG, Germany) as representative macrocyclic GBCAs. All contrast agents were dissolved in water to determine their relaxivities using six concentrations each. Different ZnCl2 concentrations were added to release the Gd3+ ions from their chelators in water and in heparin solution. The amount of Gd3+ ions released into water was quantified using the following formula: $$c_{Gd^{3+}} = \frac{R_{1,obs} - R_{1,H_2O} - c_{ZnCl_2} \cdot r_{1,ZnCl_2} -c_{GBCA} \cdot r_{1,GBCA}}{r_{1,Gd^{3+}} - r_{1,GBCA}}$$.$$$R_{1,obs}$$$ is the measured relaxation rate and $$$R_{1,H_2O} \approx 0.34 s^{-1}$$$ the relaxation rate of pure water. To calculate the amount of Gd3+ ions that transchelated to heparin, an adapted version of this formula was used, which further takes heparin and potential interaction terms into account.
Results
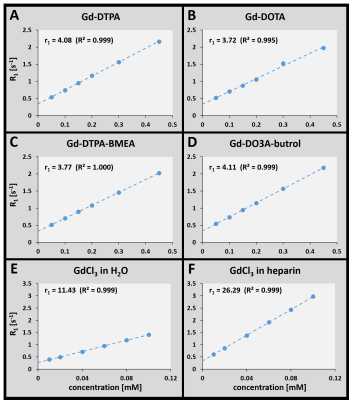
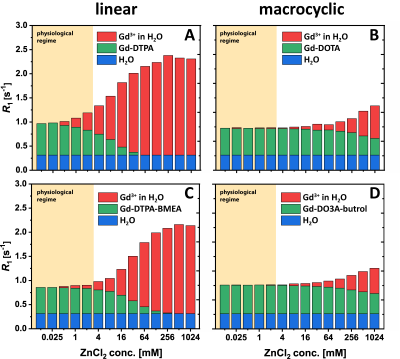
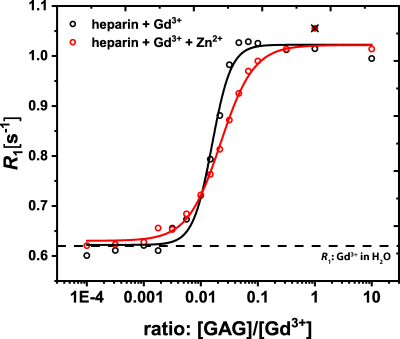
Figure 1 shows the results of the relaxivity measurements off all investigated GBCAs in water and of GdCl3 in water and heparin. Using these relaxivities, the amount of released Gd3+ ions from the GBCAs and thus the individual contributions to the observed relaxation rates of water could be quantified for different ZnCl2 concentrations (Fig. 2). For a ZnCl2 concentration of 2 mM, which is still in the physiological regime, the amount of Gd3+ ions released from both macrocyclic GBCAs is negligible (Fig 2B&D), but especially for Gd-DTPA (Fig. 2A) more than 20% of the Gd3+ ions were released. The binding potential of GAGs for Gd3+ ions was proven in a titration experiment where a constant amount of 25 µM GdCl3 was added to different amounts of heparin with (red) and w/o (black) ZnCl2 (Fig. 3). From the plateau (for ratios larger than 1), we can conclude that no free Gd3+ ions remain in solution. Further, the relaxivity of the formed Gd-heparin complexes can be estimated (from R1 in the plateau and the 25 µM GdCl3 concentration) to be in the order of 30 mM-1 s-1. This is in agreement with the relaxivity measurement shown in Fig. 1F. Using this relaxivity, the amount of Gd3+ ions that transchelate from GBCAs to GAGs and the contributions to the observed relaxation rates can be quantified. This is shown for Gd-DTPA as a representative linear GBCA in Fig. 4. For the physiological ZnCl2 concentration of 2 mM, about 22% of the Gd3+ ions were released from the DTPA chelator (Fig. 4A). Due to their high relaxivity, the resulting Gd-GAG complexes are acountable for more than 50% of the observed R1 value (Fig. 4B).Discussion
Our investigations by means of MR relaxometry allowed to quantify the amount of Gd3+ ions that are released from their chelators at different ZnCl2 concentrations within and beyond the physiological regime and we were able to determine the contributions to the observed R1 values. These experiments confirmed the higher stability of macrocyclic compared to linear GBCAs[1-3,5,6]. The titration measurements prove that GAGs have a high binding affinity for Gd3+ ions and therefore in vivo can serve as competing chelators. The relaxivity of the resulting Gd-GAG complexes was shown to be about 7 times higher compared to the investigated GBCAs (cf. Fig. 1). Due to this high relaxivity, even small concentrations of Gd3+ can have a huge effect on the observed relaxation rate, which is necessary to explain the observed hyperintensities in vivo[3].Conclusion
We were able to quantify the transchelation process of Gd3+ ions from GBCAs into glycosaminoglycans. Because GAGs are a ubiquitous component of the extracellular matrix, they should be considered as the low-tumbling, Gd3+-binding macromolecular substance with resulting high relaxivity that is needed to explain the observed long-term enhancements in vivo.Acknowledgements
No acknowledgement found.References
1) Laurent, S., Elst, L. V, Copoix, F. & Muller, R. N. Stability of MRI paramagnetic contrast media: a proton relaxometric protocol for transmetallation assessment. Invest. Radiol. 36, 115–22 (2001).
2) Taupitz, M. et al. Gadolinium-containing magnetic resonance contrast media: investigation on the possible transchelation of Gd3+ to the glycosaminoglycan heparin. Contrast Media Mol. Imaging 8, 108–16 (2013).
3) Gianolio, E., Gregorio, E. Di & Aime, S. Chemical Insights into the Issues of Gd Retention in the Brain and Other Tissues Upon the Administration of Gd-Containing MRI Contrast Agents. Eur. J. Inorg. Chem. 2019, 137–151 (2019).
4) Kanda, T., Ishii, K., Kawaguchi, H., Kitajima, K. & Takenaka, D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270, 834–41 (2014).
5) Kanda, T. et al. High Signal Intensity in Dentate Nucleus on Unenhanced T1-weighted MR Images: Association with Linear versus Macrocyclic Gadolinium Chelate Administration. Radiology 275, 803–9 (2015).
6) Radbruch, A. et al. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 275, 783–91 (2015).
Figures