0172
Developing imidazoles for performing functional MRI of kidneys1The Russell H. Morgan Department of Radiology and Radiological Science, Division of MR Research, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Department of Psychiatry, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4Department of Urology, The James Buchanan Brady Urological Institute, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 5The Russell H. Morgan Department of Radiology and Radiological Science, Division of MR Research, The Johns Hopkins University School of Medicine, F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States
Synopsis
Urinary tract obstructions (UTOs) are blockages that inhibit the flow of urine through its normal path, which can lead to kidney injury and infection. Chemical exchange saturation transfer (CEST) MRI is a fast, noninvasive molecular MRI technique which has shown promise for clinical applications. In this study, we designed and tested a series of imidazoles as CEST MRI contrast agents and tested these for performing functional kidney imaging on a UTO mouse model. The results demonstrate that CEST MRI can facilitate early detection of loss in kidney function.
INTRODUCTION
Urinary tract obstructions (UTOs) are blockages that inhibit the flow of urine through its normal path (the urinary tract), including the kidneys, ureters, bladder, and urethra. UTOs can present as either benign or serious conditions, which may lead to kidney failure if left untreated. Chemical exchange saturation transfer (CEST) MRI is a fast, noninvasive molecular MRI technique which has shown promise for clinical applications.1 CEST MRI contrast can be produced by a number of organic compounds with exchangeable protons, such as glucose, creatine, nucleic acids and peptides2, however, most organic CEST agents suffer from reduced sensitivity because of their small exchangeable proton chemical shift (< 4.0 ppm). Previously our group has reported that the intramolecular hydrogen bond helps to shift the exchangeable protons to higher ppm (> 6.0 ppm).3,4 In this study, new imidazole derivatives were designed and synthesized, and tested for detecting renal impairment on a UTO mouse model.METHOD
Synthesis: A convenient synthesis of I45DCs was designed according to the published protcol.5In vitro MRI: 20 mM of I45DCs were dissolved in 1X PBS and pH titrated to 6.0, 6.3, 6.6, 6.9, 7.2 and 7.5. The samples were kept at 37 °C during imaging. The CEST images were acquired on a Bruker 11.7 T MR scanner using a RARE (RARE=32) sequence with a CW saturation pulse length = 3s, saturation field strengths (B1) from 1.2 μT to 12.0 μT. Other parameters were: TR=10 s, TE=4.5 ms, matrix size=64×64 and slice thickness = 1.2 mm, offsets between ±12 ppm.
In vivo MRI: Healthy BALB/c mice were anaesthetized with isoflurane and catheterized via the tail vein with 100 μL of I45DCs (300 mM) in PBS (neutral pH). High resolution T2W images were acquired using a RARE sequence. CEST images were acquired using a RARE sequence with centric encoding (saturation length = 3 s at B1 = 6 μT) and RARE = 32 on a Bruker 11.7 T horizontal scanner. A two offset CEST protocol was used with offsets of 7.7 ppm and 4.5 ppm repeatedly up to 1 h 16 min after I45DCs administration. We collected 8-10 pre-injection images for each offset at the two saturation powers. Other parameters were: TR/TE: 3.49/10000 ms, Matrix size: 48×48 and axial slice, thickness 1.5 mm. For CEST MRI data processing, mean pre-injection z-spectra were subtracted from all post-injection images. Twenty images were averaged using moving average filter to generate CEST contrast maps.
RESULTS and DISCUSSION
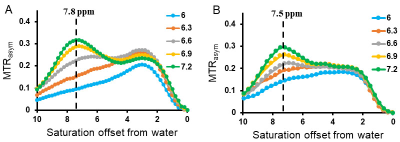
6 new imidazole CEST agents (Figure 1) were designed and synthesized. Glutamate was chosen as the side chain to increase water solubility and reduce aggregation. Among these I45DC-diGlu and I45DC-GluTyr displayed the best CEST contrast, providing significant pH sensitive contrast at 7.8 ppm and 7.5 ppm downfield from water at neutral pH (Figure 2). We selected I45DC-diGlu for testing on a UTO model and injected into mice to perform functional MRI of the kidneys. Figure 3A shows the contrast build up with time. For these mice a CEST contrast of 25% was obtained in the healthy kidney at peak contrast while only 10% is observed in the obstructed kidney (Figure 3B). In addition, some fine structure was apparent in the contrast images including differentiation between inner and outer medulla. Furthermore, no adverse effects were observed on the mice due to administration of I45DC-diGlu.CONCLUSION
We have developed a convenient synthesis for the I45DCs imidazole CEST agents. In vitro CEST contrast data of these compounds showed significant dependence on pH, which could be applied to produce pH maps. In vivo CEST MRI showed excellent delineation between unobstructed and obstructed kidneys on a UTO mouse model. This class of agent appears promising for clinical application.Acknowledgements
This project is funded by NIH P41EB024495.References
1. Vinogradov E, Sherry AD, Lenkinski RE. CEST: from basic principles to applications, challenges and opportunities. J Magn Reason. 2013; 229: 155-172.
2. Hancu I, Dixon WT, Woods M, et al. CEST and PARACEST MR contrast agents. Acta Radiol. 2010; 51: 910-923.
3. Yang X, Song X, Ray Banerjee S, et al. Salicylic acid and analogues as diaCEST MRI contrast agents with highly shifted exchangeable proton frequencies. Angew. Chem., Int. Ed. 2013; 52: 8116-8119.
4. Yang X, Song X, Ray Banerjee S, et al. Developing imidazoles as CEST MRI pH sensors. Contrast Media Mol Imaging. 2016;11(4):304-312.
5. Saudi M, Zmurko J, Kaptein S, et al. Synthesis and evaluation of imidazole-4,5- and pyrazine-2,3-dicarboxamides targeting dengue and yellow fever virus. Eur J Med Chem. 2014; 87: 529-539.
Figures