0171
High-sensitivity in vivo Contrast Agent Imaging at Ultra-low Magnetic Fields with SPIONs1Institute of Medical Physics, School of Physics, The University of Sydney, Sydney, Australia, 2A. A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 3ACRF Image X Institute, Faculty of Medicine and Health, The University of Sydney, Sydney, Australia, 4ARC Centre of Excellence for Engineered Quantum Systems, School of Physics, The University of Sydney, Sydney, Australia, 5Sydney Nano Institute, The University of Sydney, Sydney, Australia, 6Department of Physics, Harvard University, Cambridge, MA, United States, 7Harvard Medical School, Boston, MA, United States
Synopsis
MRI scanners operating at ultra-low fields (ULF) promise to reduce the cost and expand the clinical accessibility of MRI. Here, we use a 6.5 mT MRI scanner and an efficient balanced steady-state free precession MRI protocol to image superparamagnetic iron oxide nanoparticles (SPIONS) in vivo by leveraging the extremely high magnetization of SPIONs at ULF. Further, we show how positive contrast imaging of SPIONs can be performed at ULF with susceptibility-based techniques. These advances overcome a key limitation of ULF MRI by enabling high-contrast in vivo imaging of clinically safe contrast agents with short acquisition times.
Introduction
Electromagnet-based MRI scanners operating at ultra-low magnetic fields (ULF, < 10 mT) have demonstrated clinically-relevant imaging of the human brain.[1] Due to low construction costs, as well as ease of siting, ULF MRI scanners could become common screening tools in emergency medicine.[2]A key challenge in ULF MRI remains achieving contrast-to-noise ratios (CNRs) sufficient for diagnostic differentiation between tissues in a clinically acceptable imaging time.[3] Since relaxation-based contrast agents (CAs) generally modulate signal intensity, their utility is limited by the inherently low signal-to-noise ratio (SNR) in the ULF MRI regime.[4,5]
Recently, the SNR of ULF MRI has been significantly improved through the use of high-efficiency sequences, such as balanced steady-state free precession (bSSFP), that can accommodate rapid signal averaging.[6]
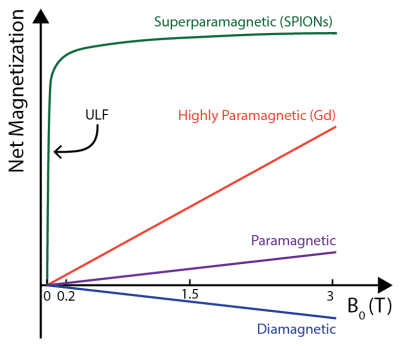
Here, we use an ULF (6.5 mT) MRI scanner and a bSSFP MRI protocol to image commercially available superparamagnetic iron oxide nanoparticles (SPIONS) in vivo. This approach generates MRI contrast due to the highly magnetized state of SPIONs at ULF (see Figure 1). Further, we take advantage of the exquisite frequency sensitivity of the bSSFP sequence to off-resonance spin precession to generate positive contrast via susceptibility effects.[7,8] These in vivo results represent the highest sensitivity CA imaging at ULF to date. Given their unique low-field properties, we expect tailored SPIONs to become the CA of choice for ULF MRI.
Methods
Highly-susceptible (HS) SPIONs were obtained from Imagion Biosystems (PrecisionMRX). FDA-approved ferumoxytol SPIONs and gadopentetic acid (Gd-DTPA; Magnevist) were obtained clinically for comparison.Under anesthesia, a tail vein catheter was placed into a 300 g male Wistar rat. A nose cone was used for general anesthesia during imaging experiments whilst oxygen saturation and cardiac/respiratory rates were continuously monitored.
Animal imaging was performed at 6.5 mT (1H = 276 kHz) in an ULF MRI scanner [1] using a custom-built probe [9] and a bSSFP sequence. Imaging parameters were: matrix size = 128x45x11, resolution = (2.0x1.6x5.9) mm3, TE/TR = 25/50 ms, α = 90° (or 20°) and NA = 60 (a 12.5 min acquisition). NA was doubled for 20° scans. Zero-filling was used to double matrix size in the slice direction.
A 1 mL bolus of CA diluted in saline to a clinical dose (5 mg/kg for SPIONs and 0.2 mL/kg for Gd-DTPA) was administered via the tail vein. Thirty minutes were allowed for biodistribution before post-injection imaging.
Results
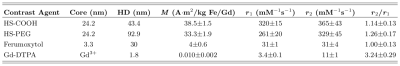
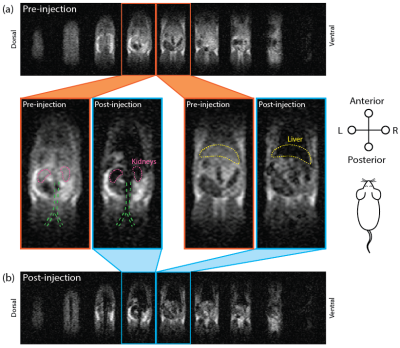
The HS-SPIONs have magnetizations at 6.5 mT that are 3850 times larger than Gd-DTPA (Fig 2). As a result, HS-SPIONs have r1/r2 relaxivities at ULF of ~300 mM-1s-1 which is more than two orders of magnitude larger than Gd-DTPA.[5]In pre-injection imaging (Fig. 3a) we measure SNRs of 23 in adipose tissue following a short scan (12.5 minutes) despite the 6.5 mT field strength. In images acquired after injection of PEGylated HS-SPIONs (Fig 3b) we observe negative contrast in highly perfused organs such as the kidneys and liver due to the uptake of SPIONs. This negative contrast arises when the CA causes T2 to become shorter than TE, which occurs at a low 100 μM threshold for HS-SPIONs. This in vivo experiment was repeated with Gd-DTPA at the maximum clinically acceptable concentration and no contrast changes were observed due to the low relaxivity of Gd-DTPA at 6.5 mT.
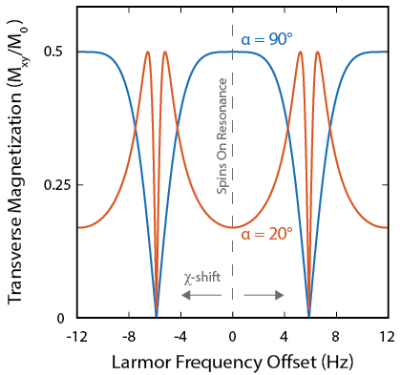
Positive contrast in MRI scans is often preferred by clinicians.[10] Varying the tip angle (α) used for bSSFP acquisition provides a mechanism for generating positive contrast from SPIONs. This mechanism is illustrated in Fig. 4, where reducing α from 90° to 20° suppresses the MRI signal from on-resonant spins and boosts the MRI signal from susceptibility-shifted spins (Fig. 4).
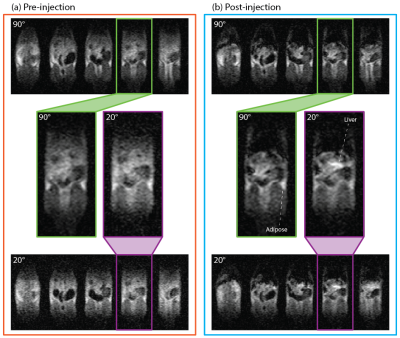
To demonstrate positive contrast SPION imaging, pre-injection anatomy scans were acquired with 90° and 20° tip angles (Fig. 5a). Contrast between tissues in these scans is qualitatively identical, as all spins are on-resonance. Following injection of HS-SPIONs, 90° and 20° bSSFP MRI scans were reacquired (Fig. 5b). While the 90° images show negative contrast in the liver and kidneys as observed in Figure 3a, the 20° images show positive contrast around regions of SPION uptake (e.g. liver) due to signal boosting of off-resonance spins.
Discussion
These results demonstrate 6.5 mT imaging of SPIONs in vivo with doses three times lower than used for clinical anemia treatments.[11] Given the common off-label use of ferumoxytol SPIONs as a high-field MRI CA, we foresee few barriers to clinical trials of our bSSFP-based ULF SPION imaging.[12]Whilst further opportunities exist to improve SNR and CNR of CA imaging at ULF (e.g. via bSSFP techniques with variable TR),[8] our current approach enables scan times that are over 40 times shorter than spin-echo T1-weighted techniques.[5] The ability to switch between negative and positive contrast regimes via tip angle will aid the unambiguous identification of CAs in clinical images.[10]
Conclusion
We have developed the most sensitive technique for CA imaging at ULF that we are aware of. This technique leverages the high magnetization of SPIONs at ULF to produce relaxivity and susceptibility-based contrast in images acquired in vivo with clinically feasible doses and timeframes. Given the biocompatibility of SPIONs, these results will likely lead to new clinical applications of ULF MRI in emergency medicine.Acknowledgements
The authors are grateful to Fanny Herisson, Neha Koonjoo and Nicholas Rotile for assistance with animal experiments. The authors thank Imagion Biosystems for providing materials. Z.K. acknowledges funding from an Australian Academy of Technological Sciences and Engineering (ATSE) Global Connections Fund Bridging Grant.References
[1] M. Sarracanie, C.D. Lapierre, N. Salameh, D.E.J. Waddington, T. Witzel, M.S. Rosen, Sci Rep 5 (2015) 15177.
[2] M. Espy, A. Matlashov, P. Volegov, J Magn Reson 229 (2013) 127–141.
[3] R.E. Sepponen, J.T. Sipponen, A. Sivula, J Comput Assist Tomogr 9 (1985) 237–241.
[4] D.E.J. Waddington, M. Sarracanie, H. Zhang, N. Salameh, D.R. Glenn, E. Rej, T. Gaebel, T. Boele, R.L. Walsworth, D.J. Reilly, M.S. Rosen, Nat Commun 8 (2017) 15118.
[5] X. Yin, S.E. Russek, G. Zabow, F. Sun, J. Mohapatra, K.E. Keenan, M.A. Boss, H. Zeng, J.P. Liu, A. Viert, S.-H. Liou, J. Moreland, Sci Rep 8 (2018) 11863.
[6] M. Sarracanie, B.D. Armstrong, J. Stockmann, M.S. Rosen, Magn Reson Med 71 (2013) 735–745.
[7] R. Dharmakumar, I. Koktzoglou, D. Li, Phys Med Biol 51 (2006) 4201–4215.
[8] T. Çukur, M. Yamada, W.R. Overall, P. Yang, D.G. Nishimura, Magn Reson Med 63 (2010) 427–437.
[9] D.E.J. Waddington, M. Sarracanie, N. Salameh, F. Herisson, C. Ayata, M.S. Rosen, NMR Biomed 31 (2018) e3896.
[10] C. Lin, S. Cai, J. Feng, J Nanomater 2012 (2012) 734842.
[11] A.M. Muehe, D. Feng, R. Von Eyben, S. Luna-Fineman, M.P. Link, T. Muthig, A.E. Huddleston, E.A. Neuwelt, H.E. Daldrup-Link, Invest Radiol 51 (2016) 221–227.
[12] V. Trujillo-Alonso, E.C. Pratt, H. Zong, A. Lara-Martinez, C. Kaittanis, M.O. Rabie, V. Longo, M.W. Becker, G.J. Roboz, J. Grimm, M.L. Guzman, Nat Nanotechnol 14 (2019) 616–622.
[13] P. Cantillon-Murphy, L.L. Wald, M. Zahn, E. Adalsteinsson, NMR Biomed 22 (2009) 891–897.
Figures