0164
Variation of In Vivo Anisotropic MRE Metrics in Corpus Callosum: Effect of Aging1Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, United States, 2Beckman Institute, Urbana, IL, United States, 3Department of Mechanical Science and Engineering, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 4Department of Mechanical and Aerospace Engineering, Rutgers University, New Brunswick, NJ, United States
Synopsis
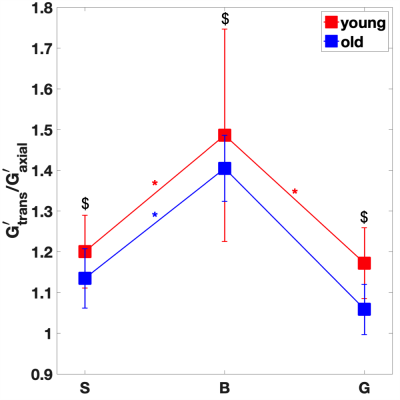
Healthy aging involves local variations in viscoelastic shear properties of the brain. We employ high-resolution, multi-excitation MRE and a novel anisotropic inversion scheme (iTI) to extract local shear anisotropic moduli in vivo. The ratio of transverse to axial moduli, a new MRE metric, remains greater than 1 along the splenium, body and genu regions of the corpus callosum for both young and old subjects. This metric peaks in the body region and decreases with age throughout the corpus callosum.
INTRODUCTION
Using isotropic tissue models for inversion, prior MRE studies have shown that there is a general decrease of the human brain stiffness during healthy aging [1-3]. A class of anisotropic inversion methods rely on directional filtering of the displacement fields in judiciously chosen regions of the brain, followed by direct extraction of certain components of the stiffness tensor, which are assumed uniform in the chosen regions [4,5]. Romano et al. [4] found that both anisotropic shear moduli decrease in the corticospinal tract of amyotrophic lateral sclerosis patients, while Kalra et al. [5] reported a negative correlation of anisotropic to isotropic stiffness averaged over the corpus callosum.Seeking to explore the effect of age on the mechanical anisotropy of the brain, we employ a novel iterative inversion scheme based on a transversely isotropic tissue model to extract local viscoelastic moduli in the human corpus callosum. Specifically, we incorporate the data obtained in a multi-excitation MRE and diffusion tensor imaging (DTI) study of the brain [6,7] in our iterative scheme to estimate two sets of moduli, corresponding to shear in the transverse and axial planes relative to the local axon direction. In this study we compare the average moduli values in the splenium, body, and genu regions of the corpus callosum between two cohorts of healthy young and old adults.
METHODS
A previous study [7] generated anterior-posterior (AP) excitation MRE, and left-right (LR) excitation brain MRE data for 7 young (24-32 years old) and 4 older (55-76 years old) males. Using a 3D multislab, multishot spiral MRE sequence [8], 3D complex displacement field data was collected at 50 Hz with 2 x 2 x 2 mm3 isotropic spatial resolution. The isotropic viscoelastic properties were estimated for both AP and LR data (see Figure 1(a)) using nonlinear inversion (NLI). Additionally, matched field-of-view and resolution (relative to MRE) diffusion tensor imaging (DTI) and high-resolution anatomical MPRAGE at 0.9 x 0.9 x 0.9 mm3 isotropic resolution (TR/TI/TE = 2000/900/2.2 ms) were acquired, to register the subjects to the MNI JHU white matter atlas.A novel inverse Transversely Isotropic (iTI) scheme has been introduced in [9]. Under iTI, we use both AP and LR data in conjunction with co-registered DTI data, to extract both shear moduli based on a transversely isotropic constitutive tissue model. This model involves a linear stress-strain relationship, nearly incompressible medium with Young’s moduli ratio of 2.7 [10], and Poisson’s ratio of 0.499. For each voxel, iTI produces a pair of complex shear stiffness moduli, one transverse (G*trans) and one axial (G*axial), cf. Figure 1(b). Referring to Figure 1(c), we define multiple ROIs centered in the splenium, body, and genu of the corpus callosum (CC) of each subject.
RESULTS AND DISCUSSION
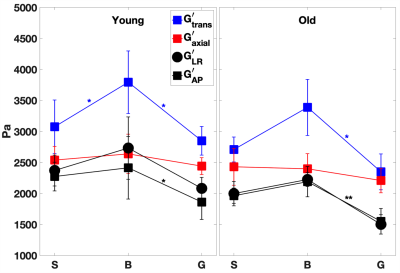
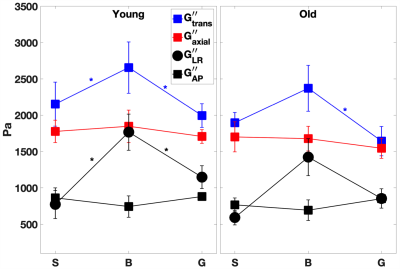
For the young cohort, Figures 2-3 indicate that both transverse storage (G’trans) and loss (G”trans) moduli vary significantly (P < 0.05) across the CC, with the highest values attained at the CC body. For the older cohort, the trend is similar, with significance only between body and genu. The variation in NLI moduli across the CC is not as significant. We note that there is a trend for all 4 anisotropic moduli to decrease with age, although not statistically significant.In order to explore the effect of age, the shear anisotropy metric (G’trans/ G’axial) is plotted in Figure 4. The variation of this metric is significant (P < 0.05) across the CC, again with the highest value at the body. Moreover, the anisotropy metric decreases significantly with age (P < 0.01). We also observe that this metric remains above 1 in the CC, consistent with findings in corticospinal tract [4]. Extending the multiphase micromechanics model in [11], we propose that this anisotropy is consistent with the fact that the shear stiffness of axons is higher than that of the glial matrix.
CONCLUSION
The novel inversion scheme iTI has enabled the extraction of local in vivo anisotropic MRE metrics in three regions across the corpus callosum for two healthy age groups. By introducing the mechanical shear anisotropy metric, iTI accentuates the difference between young and old cohorts.The additional information provided by iTI in terms of transverse and axial metrics increases the specificity of brain MRE. This enhanced specificity allows visualization of significant local regional differences in shear properties across the splenium, body and genu of the corpus callosum for both young and old subjects.
Acknowledgements
Support was provided by NSF Grants CMMI-1437113 and CMMI-1762774. Partial support was provided by the Biomedical Imaging Center of the Beckman Institute for Advanced Science and Technology at the University of Illinois at Urbana-Champaign (UIUC), NIH Grant R01-EB018230, and NIH/NIBIB Grant R01-EB001981.References
[1] Sack I, Beierbach B, et al. The impact of aging and gender on brain viscoelasticity. NeuroImage. 2009;46:652–657.
[2] Arani A, Murphy MC, et al. Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. NeuroImage. 2015;111, 59–64.
[3] Murphy MC, Huston J, Ehman RL. MR elastography of the brain and its application in neurological diseases. Neuroimage. 2019; 187:176-183.
[4] Romano A, Guo J, et al. In vivo waveguide elastography: Effects of neurodegeneration in patients with amyotrophic lateral sclerosis. Magn. Reson. Med. 2014;72(6):1755-1761.
[5] Kalra, P, Raterman, B, et al. 2019. Magnetic resonance elastography of brain: Comparison between anisotropic and isotropic stiffness and its correlation to age. Magn. Reson. Med. 82, 671-679.
[6] Anderson, AT, Van Houten, EEW, et al. Observation of direction-dependent mechanical properties in the human brain with multi-excitation MR elastography. JMBBM. 2016;59, 538–546.
[7] Anderson, AT, Johnson, CL, et al., Multi-Excitation MRE in Aging Human Brain. 26th Annual Meeting of ISMRM. 2018.
[8] Johnson, CL, Holtrop, JL, et al. 3D multislab, multishot acquisition for fast, whole-brain MR elastography with high signal-to-noise efficiency. Magn. Reson. Med. 2014; 71, 477–485.
[9] Gallo, NR, Cahoon, SM, et al. Multi-Excitation MRE in Aging Human Brain: Estimation of Direction-Dependent Mechanical Properties. 28th Annual Meeting of ISMRM. 2019; p. 1058.
[10] Feng Y, Okamoto RJ, et al. Measurements of mechanical anisotropy in brain tissue and implications for transversely isotropic material models of white matter. JMBBM. 2013;23:117–132.
[11] Sullivan, DJ, Wu, X , et al. Sensitivity Analysis of Effective Transverse Viscoelastic and Diffusional Properties of Tissue with Myelinated Axons, NeuroImage, under review.
Figures