0157
Simultaneous QSM and MR Elastography of the Brain Using Spiral Staircase1Department of Radiology, Mayo Clinic, Rochester, MN, United States
Synopsis
This work presents a new feasibility to extract tissue susceptibility from gradient-echo MRE data and enables simultaneous QSM and MRE in a single scan. The proposed method builds on a new spiral staircase acquisition which enables high resolution often required by QSM using inherently improved through-plane parallel imaging. In-plane parallel imaging, constrained reconstruction and deblurring method are also integrated to generate high quality spiral images for QSM and MRE processing. In vivo experiment results demonstrate the capability of proposed method in producing high quality tissue susceptibility along with shear stiffness maps from a single 5-minute scan.
INTRODUCTION
Quantitative susceptibility mapping (QSM) has been recognized as a unique biomarker of tissue magnetic susceptibility. MR Elastography (MRE) has been widely used as a powerful tool for in vivo mapping of tissue mechanical property. QSM and MRE therefore, provide complementary information useful for many research and clinical applications in diagnosis and characterization of neurological disorders1-6. Currently, standard QSM and MRE of the brain are performed in separate spin-echo (SE) and gradient-echo (GRE) scans respectively with different resolution considerations, thus no simultaneous acquisitions, to the best of our knowledge, have ever been attempted. This work reports the feasibility of simultaneous QSM and MRE from a single 3D GRE scan using spiral staircase (SSC) acquisition. This is possible by exploiting the fact that both the static tissue phase containing susceptibility information and motion induced phase for stiffness inversion are simultaneously encoded in a gradient-echo acquisition. The new SSC acquisition, parallel imaging and processing scheme are then integrated to enable simultaneous QSM and MRE with high resolution and high SNR. In vivo human experiments have shown that a single GRE-SSC scan can be performed in 5 minutes at a 1.6×1.6×1.6mm3 resolution and produce high quality susceptibility and stiffness maps.METHODS
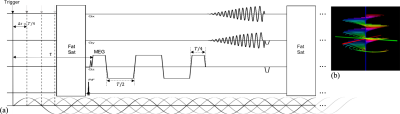
1) Data Acquisition: The proposed pulse sequence and SSC acquisition are illustrated in Fig. 1. The new SSC trajectory contains M groups of N spiral arms (each rotates by n*360°/N). Each set of M arms (all at the same angle about kz) remain sampled at the Nyquist distance Δkz. Each group of N spiral arms uniformly distribute over Δkz with a shift of n*Δkz/N. This shift results in a linear phase variation as a function of slice coordinate and leads to improved through-plane parallel imaging with much less SNR penalty. To achieve the resolution to the level often required by QSM, both through-plane and in-plane parallel imaging are exploited. To enhance the displacement-SNR in MRE, a motion encoding gradient (MEG) of a 2 wave period (T) duration is adopted to boost the encoding efficiency, while still maintaining a short TE of 35ms approximately optimal for both QSM and MRE at 3T in the brain7.2) Image Reconstruction: Spiral images obtained from each MRE phase and MEG direction were reconstructed by solving the following optimization problem:$$\hat{\rho}=\underset{\rho}{\operatorname{argmin}} \sum_{l=1}^{L}\left \| G_{xy}^{H}FP_zS_l\rho - d_l \right \|^2_2 + \lambda R(\rho)$$where $$$\rho$$$ denotes the target image function, $$$S_l$$$ the $$$l$$$-th coil sensitivity profile, $$$P_z$$$ the linear phase compensation term for SSC along z, $$$F$$$ the Fourier encoding operator, $$$G_{xy}$$$ the 2D gridding operator and $$$H$$$ the Hermitian conjugate operator. $$$d_l$$$ contains the SSC data from the $$$l$$$-th coil. $$$R(·)$$$ is a regularization function (e.g., Tikhonov regularization in this study) incorporating prior information with parameter $$$\lambda$$$ controlling the strength of the prior. After reconstruction, spatial blurring caused by off-resonance is corrected using a conjugate gradient deblurring method7 with a known $$$B_0$$$ map. Both the sensitivity and $$$B_0$$$ maps were obtained from a low-resolution prescan.
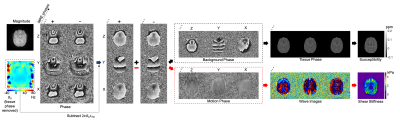
3) Data Processing: The data processing scheme for simultaneous QSM and MRE is illustrated in Fig. 2. The phase caused by the main field inhomogeneity (i.e., $$$2πB_0t_{TE}$$$) is first subtracted from all the spiral images. To generate proper phase information for QSM and MRE processing, phase from reverse MEG directions (e.g., ±z) are added and subtracted, yielding the static phase and motion phase respectively. With proper phase unwrapping9, the static phase is used to extract the tissue phase within the brain region using V-SHARP10. After average over all the MRE phases and MEG directions, the tissue phase is exploited to reconstruct tissue susceptibility using MEDI11. On the other hand, given the curl of the motion phase determined12, the shear stiffness can be estimated via direct inversion of the Helmholtz equation13.
RESULTS
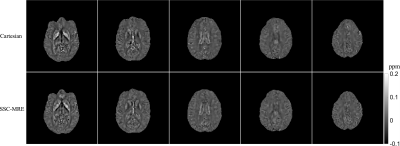
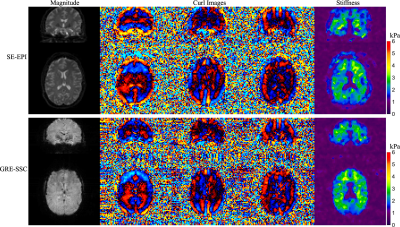
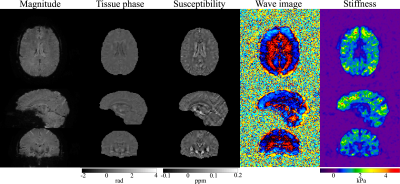
In vivo experiments were performed on a 3T Phillips system with a 14-channel receiver head coil with institutional approved IRB. SSC data were acquired using the proposed method at a resolution of 1.6×1.6×1.6mm3 in 5 minutes (FOV 240×240×120mm3, TR/TE 67/35 ms, FA 23°, 1.2x slice oversampling, 2× through-plane undersampling, 4 spiral shots, 16 ms spiral readout, wave frequency 60Hz, 4 MRE phases and 6 MEG directions). For comparison, a 3D single-echo gradient-echo Cartesian QSM scan and a standard 2D single-shot spin-echo EPI MRE scan were performed.Results generated from the proposed method and separate QSM and MRE scans were compared in Fig. 3 and Fig. 4. The proposed method provides high-quality susceptibility images and high-SNR shear wave and stiffness maps comparable to the standard methods within a single scan. Figure 5 summarized all the results of the proposed method obtained in 5 minutes, demonstrating the powerful capability of the proposed technique for simultaneous susceptibility and mechanical property mapping.
CONCLUSION
A new technique for simultaneous QSM and MR Elastography of the brain is proposed. The technique combines a new spiral staircase acquisition, parallel imaging, constrained reconstruction and processing schemes to provide a new capability to obtain QSM and high resolution MRE results from a single scan. Future work may include further increase the resolution of QSM by using compressed sensing.Acknowledgements
This work is supported in part by a research funding from Philips.References
1. Acosta-Cabronero J, Williams GB, Cardenas-Blanco A, Arnold RJ, Lupson V, Nestor PJ. In vivo quantitative susceptibility mapping (QSM) in Alzheimer’s disease. PLoS One 2013;8:e81093.
2. Langkammer C, Liu T, Khalil M, Enzinger C, Jehna M, Fuchs S, Fazekas F, Wang Y, Ropele S. Quantitative susceptibility mapping in multiple sclerosis. Radiology 2013;267:551–559.
3. Lotfipour AK, Wharton S, Schwarz ST, Gontu V, Schafer A, Peters AM, Bowtell RW, Auer DP, Gowland PA, Bajaj NPS. High resolution magnetic susceptibility mapping of the substantia nigra in Parkinson’s disease. J Magn Reson Imaging 2012;35:48–55.
4. Murphy MC, Huston J, Jack CR, Glaser KJ, Manduca A, Felmlee JP, Ehman RL. Decreased brain stiffness in Alzheimer’s disease determined by magnetic resonance elastography. J Magn Reson Imaging 2011;34:494–498.
5. Streitberger K-J, Sack I, Krefting D, Pfuller C, Braun J, Paul F, Wuerfel J. Brain viscoelasticity alteration in chronic-progressive multiple sclerosis. PLoS One 2012;7:e29888.
6. Freimann FB, Streitberger K-J, Klatt D, Lin K, McLaughlin JR, Braun J, Sprung C, Sack I. Alteration of brain viscoelasticity after shunt treatment in normal pressure hydrocephalus. Neuroradiology 2012; 54:189–196.
7. Wu B, Li W, Avram AV, Gho SM, Liu C. Fast and tissue‐optimized mapping of magnetic susceptibility and T2* with multiecho and multi‐shot spirals. NeuroImage 2012;59:297–305.
8. Wang D, Zwart NR, Pipe JG. Joint water–fat separation and deblurring for spiral imaging. Magn Reson Med. 2018;79(6):3218–3228
9. Li W, Wu B, Liu C. Quantitative susceptibility mapping of human brain reflects spatial variation in tissue composition, NeuroImage. 2011;15; 1655:1645.
10. Wu B, Li W, Liu C. Whole brain susceptibility mapping using compressed sensing. Mag. Res. Med. 2012;67(1):137.
11. Liu J, Liu T, de Rochefort L, Ledoux J, Khalidov I, Chen W, Tsiouris AJ, Wisnieff C, Spincemaille P, Prince MR, Wang Y. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage 59: 2560-2568.
12. Glaser KJ, Ehman R. MR Elastography Inversions Without Phase Unwrapping. ISMRM, 2009.
13. Manduca A, Oliphant TE, Dresner MA, Mahowald JL, Kruse SA et al. Magnetic resonance elastography: Non-invasive mapping of tissue elasticity. Med Image Anal 2001; 5: 237-254
Figures