0155
Morphology Enabled Quantitative Conductivity–Susceptibility Mapping with B1 and B0 Estimation from Complex Multi-echo Gradient Echo Signal1Graduate School of Information Science and Technology, The University of Tokyo, Tokyo, Japan, 2Radiology, Weill Cornell Medical College, New York, NY, United States, 3Biomedical Engineering, Cornell University, Ithaca, NY, United States
Synopsis
We propose a simultaneous conductivity and susceptibility reconstruction method by estimating B1 phase and B0 distributions from a multi-echo gradient echo (mGRE) signal. B1 phase and B0 maps are simultaneously determined by applying nonlinear least squares method on the complex signal equation of the mGRE signal. The poor conditioned inversion of field (B1/B0) to source (conductivity/susceptibility) is regularized using anatomical information. This morphology enabled quantitative conductivity and susceptibility mapping (QCSM) was performed on healthy subjects and patients with brain tumors. Our preliminary in-vivo experiments demonstrated that the proposed QCSM method can reconstruct conductivity and susceptibility from a single mGRE acquisition.
Introduction
For studying electromagnetic tissue properties [1], EPT [2] reconstructs conductivity from transceive B1 phase data without B1 magnitude mapping, and QSM [3] retrieves tissue’s magnetic susceptibility from B0 field data typically estimated from multi-echo GRE (mGRE) signal. In a traditional phase based EPT, the transceive B1 phase is obtained from spin echo (SE) acquisition, which is free from B0 inhomogeneity effect and reflects only B1-related phase. Several studies show that transceiver B1 phase can also be estimated from mGRE signal by extrapolating phase evolution along echo time [4]. In this paper, we estimate B1 phase and B0 field distributions simultaneously from mGRE signal by applying nonlinear least squares on the complex signal equation [5], which can achieve the maximum likelihood estimation.Method
Since EPT assumes that both transmit and receive B1 magnitude fields are sufficiently homogeneous to retrieve conductivity from B1 phase information, individual coil’s data were properly combined to minimize receive B1 magnitude variation [6,7] as follows. Receiver coil sensitivities $$$B_{1,j}^{-}$$$ were first estimated using ESPIRiT method [8], and then combination coefficients $$$c_{j}(\boldsymbol{r})$$$ were determined by minimizing combined receive B1 field variation:$$c_{j}(\boldsymbol{r})=\mathrm{argmin}_{c_{j}(\boldsymbol{r})}\|\sum_{j = 1}^{N}B_{1,j}^{-}c_{j}(\boldsymbol{r})-1\|_{2}^{2}+\lambda\sum_{j = 1}^{N}|c_{j}(\boldsymbol{r})|^{2},$$
where norm was taken inside the neighboring region of each point . Next, B1 phase as well as B0 field were estimated according to Gaussian noise model in complex mGRE data:
$$\phi,\Delta B_{0}=\mathrm{argmin}_{\phi,\Delta B_{0}}\|S(T_{E})-\rho\exp(-R_{2}^{\ast}T_{E})\exp(\mathrm{i}(\phi-\gamma\Delta B_{0}T_{E}))\|_{2}^{2},$$
where $$$\rho$$$ is proton density, $$$R_{2}^{\ast}$$$ is relaxation rate, $$$\gamma$$$ is gyromagnetic ratio, and $$$T_{E}$$$ is echo time. The summation is over the all echoes. Minimization was conducted by the Levenberg–Marquardt method. To compare B1 phase estimated from mGRE with SE phase, two FSE images with opposite readout gradients were also acquired and averaged to yield the gold standard phase image.
Once coil-combined B1 phase image was obtained, its Laplacian was calculated using Savitzky-Golay filter, which is based on the second order weighted polynomial fitting in each local region around the voxel of interest. Kernel size was 15x15x5 voxels and the weighting factor was determined from magnitude image as follows: $$$w(\boldsymbol{r}) = G(|I(\boldsymbol{r}) - I(\boldsymbol{r}_{0})|)$$$, where $$$G$$$ represents Gaussian function [7]. Finally, conductivity $$$\sigma$$$ was reconstructed as follows:
$$\sigma=\mathrm{argmin}_{\sigma}\|\sigma-\Delta\phi/(2\omega_{0}\mu_{0})\|_{2}^{2}+\lambda\|M(\nabla I)\nabla\sigma\|_{1},$$
where $$$M(\nabla I)$$$ represents the mask that removes boundaries of different anatomical regions and is used in morphology enabled dipole inversion (MEDI) method [9] in QSM. QSM was also reconstructed using nonlinear MEDI with automatic uniform cerebrospinal fluid zero reference (MEDI+0) [10]. This electromagnetic tissue property estimation from mGRE complex signal is referred to as quantitative conductivity and susceptibility mapping (QCSM).
MRI acquisitions were performed on 5 healthy human subjects and brain data were obtained using 2D FSE and 3D mGRE in a 3T clinical scanner (MR750, GE Healthcare, Waukesha, WI). A 32-channel head coil was used as the receiver coil. The imaging parameters for mGRE were as follows: TR: 53.2 ms, first TE: 4.4 ms, echo spacing: 4.9 ms, flip angle: 15 deg; and for FSE : TR: 5350 ms, eff. TE: 87.9 ms, echo train length: 24, flip angle: 111 deg, NEX: 2. FOV was 240x240x144 mm3 and voxel size was 0.4688x0.4688x3 mm3 for both sequences. QCSM was generated from mGRE data. We also tested the proposed QCSM method with 5 tumor patients’ mGRE data.
Results and Discussion
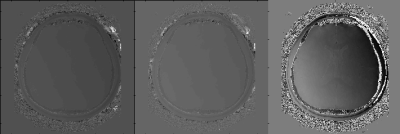
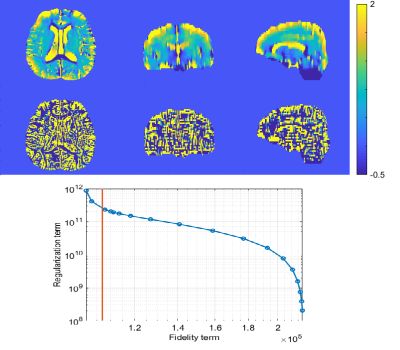
Figure 1 shows the estimated transceiver B1 phase of one representative subject. The estimated B1 phase maps were similar in both linear extrapolation method (left) and in the proposed nonlinear least square method (center). Although B1 phase is less smooth compared with SE phase (right) in both linear and nonlinear methods, the proposed method yielded relatively stable results compared with the linear extrapolation method. This is because the proposed method correctly accounted for the noise distribution and achieves maximum likelihood estimation.Figure 2 shows the conductivity maps reconstructed from B1 phase estimated by the nonlinear least square method. We varied the regularization parameter and chose the one that maximizes the curvature of L-curve plot shown in Fig.2 (bottom). When no regularization is adopted (middle), the conductivity map is suffered from severe noise. When the anatomical image is utilized as a regularizer (top), the conductivity results are less noisy and anatomical structures are discernible.
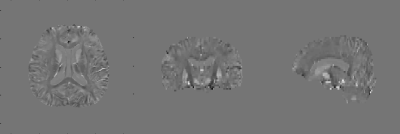
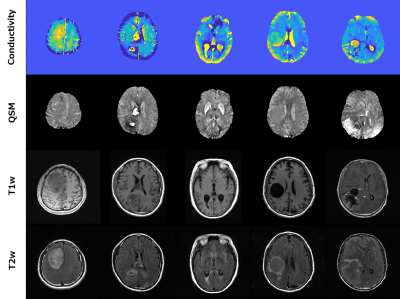
Simultaneously, we obtained B0 field map by solving Eq.2 and reconstructed QSM (Fig.3). This means that electromagnetic tissue properties can be successfully obtained from a single mGRE acquisition. Figure 4 shows the reconstructed conductivity and susceptibility maps along with T1w (3rd row) and T2w (bottom row) images for 5 tumor patients’ data. The structures of lesions are consistent between conductivity and susceptibility maps, but the conductivity maps have higher values in all cases. Therefore, conductivity maps can give additional pathological information.
Conclusion
We describe a simultaneous quantitative conductivity and susceptibility mapping (QCSM) method by estimating B1 phase and B0 field distributions from multi-echo gradient echo (mGRE) signal. B1 phase and B0 filed maps are simultaneously estimated by applying nonlinear least square method on the complex signal equation of the multi-echo GRE signal. Then, conductivity and susceptibility are reconstructed by incorporating anatomical information as a regularizer. QCSM is feasible and promising for studying electromagnetic tissue properties in healthy brains and in brains with tumors using a single mGRE acquisition.Acknowledgements
No acknowledgement found.References
- Tobias Voigt, Ulrich Katscher, and Olaf Doessel, Magnetic Resonance in Medicine, 2011; 66(2): 456–466.
- Ulrich Katscher, Cornelius A.T. van den Berg, NMR in Biomedicine, 2017; 30(8): 1–15.
- Yi Wang and Tian Liu, Magnetic Resonance in Medicine, 2015; 73(2): 82–101.
- Dong-Hyun Kim, Naraeand Choi, Sung-Min Gho, Jaewookand Shin, and Chunlei Liu, Magnetic Resonance in Medicine, 2014; 71(3): 1144–1150.
- Motofumi Fushimi, Pascal Spincemaille, and Yi Wang, 5th International Workshop on MRI Phase Contrast & Quantitative Susceptibility Mapping, 2019.
- Joonsung Lee, Narae Choi, Jaewook Shin, and Dong-Hyun Kim, Proc. Intl. Soc. Magnetic Resonance in Medicine 21, 2013; p. 4179.
- Joonsung Lee, Jaewook Shin, and Dong-Hyun Kim, Magnetic Resonance in Medicine, 2016; 76(2): 530–539.
- Martin Uecker, Peng Lai, Mark J. Murphy, Patrick Virtue, Michael Elad, John M. Pauly, Shreyas S. Vasanawala, and Michael Lustig, Magnetic Resonance in Medicine, 2014; 71(3): 990–1001.
- Tian Liu, Cynthia Wisnieff, Min Lou, Weiwei Chen, Pascal Spincemaille, and Yi Wang, Magnetic Resonance in Medicine, 2013; 69(2): 467–476.
- Zhe Liu, Pascal Spincemaille, Yihao Yao, Yan Zhang, and Yi Wang, Magnetic Resonance in Medicine, 2018; 79(2): 2795–2803.
Figures