0146
Imaging blood-brain barrier disruption caused by CD19 based CAR-T cell immunotherapy1Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Center for Cellular Immunotherapies, University of Pennsylvania, Philadelphia, PA, United States, 3Sidra Medicine, Doha, Qatar, 4LARC, Qatar University, Doha, Qatar
Synopsis
With the advent of clinically effective CD19 based chimeric antigen receptor (CAR) T cell immunotherapies, there are incidents of associated neurotoxicity. In this study, we report the use of gadolinium enhanced MRI to image BBB disruption caused by CD1928z CAR-T cell on target action against brain pericytes of immunodeficient non-tumor bearing NSG mice. Pericytes are mural cells that wrap endothelial cells and are critical for maintaining blood-brain-barrier (BBB) integrity and express CD19. The MRI results were also found to corroborate with the Evans blue dye BBB permeability assay.
Introduction
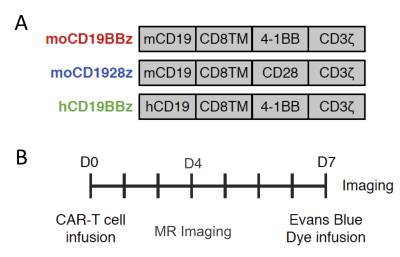
CD19-directed immunotherapies are clinically effective for treating B-cell lymphomas but also display a high incidence of associated neurotoxicity1-3. Subsets of patients treated with CD19-directed chimeric antigen receptor (CAR) T cells or CD19/CD3 bispecific T-cell engager (BiTE) antibodies display severe neurotoxicity, including cases of fatal cerebral edema associated with T cell infiltration into the brain. The pathophysiology of this neurotoxicity remains unclear, yet its understanding is critical for the development of safer CAR and BiTE therapies. Here we report that a small population of pericytes, mural cells that wrap endothelial cells and are critical for maintaining BBB integrity, in brain expresses CD19 and contributes to CD19-specific CAR-T-induced neurotoxicity4,5. We interrogate the contribution of CD19+ pericytes as targets for anti-CD19 CAR-T related neurotoxicity using the immunodeficient mouse model. We generated CD28-based or 4-1BB-based CAR-T cells specific for either murine CD19 (1D3 scFv, mCD1928z and mCD19BBz) or human CD19 (FMC63 scFv, hCD19BBz) (Figure 1A). Following CD19-specific CAR-T cell infusion, CAR-T cells disrupt the BBB through a B cell-independent mechanism. Furthermore, these data provide insights into the underlying mechanism of neurotoxicity in CD19-directed therapies and demonstrate the potential value of a single-cell human atlas for cell therapy design.Methods
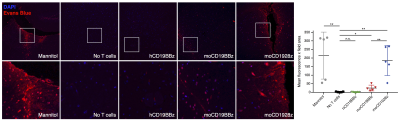
NOD/scid/IL2rg (NSG) mice were housed in the vivarium under BSL2 and pathogen-free conditions. 1-3x107 T cells with 20% normalized CAR+ expression were administered mice intravenously. The experimental design followed is depicted in Figure 1B. For the studies, mice were divided into 5 groups: 1. Mannitol, 2. No T-cell, 3. mCD1928z, 4. mCD19BBz and 5. hCD19BBz.BBB permeability assays - Seven days after CAR-T cell infusion, mice were injected intravenously with Evans Blue dye EBD +/- mannitol6,7. EBD binds to albumin, which remains in the bloodstream in normal physiologic conditions. When the BBB is disrupted, small proteins, such as albumin, can cross. Thirty minutes after EBD infusion, mice were euthanized; brain tissues were excised and fixed in 10% formalin solution. 5µm thick sections of fixed brains were analyzed by fluorescence microscopy.
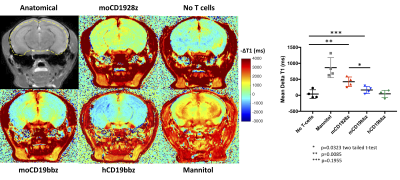
High-resolution in vivo brain MRI - MRI experiments were performed 4 days post-CAR-T cell infusions. Mice were anesthetized by using isoflurane (0.5-1.5% in 1 liter/min air). The lateral tail vein was cannulated for administration of gadodiamide (287 mg/ml) or mannitol (25% w/v). Mice were transferred to a 9.4T horizontal bore small animal MR scanner (Bruker, Billerica, MA) and placed in a 20 mm diameter commercial quadrature proton coil (m2m Imaging Corp., Cleveland, OH). T1-mapping was performed pre- and post-gadolinium injection on 0.7 mm thick coronal slice in the mid-brain using saturation recovery protocol with following parameters, averages = 2, field of view = 20×20 mm2, matrix size = 128×128, TE = 8 ms, TRs=200, 500, 800, 1200, 1500, 4000 and 9000 ms, scan time 30 minutes. Following the T1 acquisition, gadolinium was administered via tail vein as described previously8. T1 map was generated using the image sequence analysis tool in Paravision 6.0.1 by the exponential fitting of the signal recovery data. An in-house MATLAB script was used to quantify the DT1 (T1Post - T1Pre) caused by Gd retention in the brain.
Results and Discussion
Mice treated with mannitol displayed EBD fluorescence indicative of BBB extravasation, in contrast with mice receiving no treatment as well as mice receiving anti-human CD19 CAR-T cells (hCD19BBz) (Figure 2). Mice treated with anti-murine mCD19BBz cells, and to a higher extent, anti-murine mCD1928z cells, displayed BBB extravasation, the cortical regions displayed the greatest extravasation (Figure 2). This observation is consistent with the occurrence cortical cytotoxic edema observed in the rare clinical cases of fatal neurotoxicity after CD19 targeting CAR-T cell therapy2. Moreover, this disruption is more evident in CD28-based CAR-T cells compared to 4-1BB-based CAR-T cells, in agreement with clinical observations of greater neurotoxicity with CD28-based CAR-T cells. For the MRI studies, the anatomical reference image with selected ROI contour used to calculate -DT1 for each group is shown (Figure 3A). We found a statistically significant difference in BBB leakage between the “No T cell group” and the mCD1928z group and between the mCD1928z and mCD19BBz groups as well (n=4 per group) (p=0.0085 and p=0.0323 respectively with two-tailed t-tests) (Figure 3B). There was no statistical difference between the No T cell group and the mCD19BBz groups (p=0.1955).Indeed, the presence of CD19 expression in pericytes may explain the higher rate of neurologic events in CD19-targeting CAR-T cell trials, compared with other CAR-T therapies. Our findings also caution the use of intrathecal or intraventricular administration of anti-CD19 CAR-T cells for refractory primary CNS high-grade lymphoma where CAR-T cell activation could be triggered not only by the tumor itself but also by brain pericytes, especially in a patient population already at risk for leukoencephalopathy after chemotherapy and whole-brain radiotherapy. Finally, this research highlights the utility of developing a comprehensive human single-cell atlas as a resource for developing targeted clinical therapies, where putative target antigens could be identified and screened for specific expression on the target tissue.
Acknowledgements
This project was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institute of Health under award number P41EB015893.References
1. Goebeler, M.E., Knop, S., Viardot, A., Kufer, P., Topp, M.S., Einsele, H., Noppeney, R., Hess, G., Kallert, S., Mackensen, A., et al. (2016). Bispecific T-Cell Engager (BiTE) Antibody Construct Blinatumomab for the Treatment of Patients With Relapsed/Refractory Non-Hodgkin Lymphoma: Final Results From a Phase I Study. J Clin Oncol 34, 1104-1111.
2. Gust, J., Hay, K.A., Hanafi, L.A., Li, D., Myerson, D., Gonzalez-Cuyar, L.F., Yeung, C., Liles, W.C., Wurfel, M., Lopez, J.A., et al. (2017). Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov 7, 1404-1419.
3. Nagorsen, D., Kufer, P., Baeuerle, P.A., and Bargou, R. (2012). Blinatumomab: a historical perspective. Pharmacol Ther 136, 334-342
4. Armulik, A., Genove, G., Mae, M., Nisancioglu, M.H., Wallgard, E., Niaudet, C., He, L., Norlin, J., Lindblom, P., Strittmatter, K., et al. (2010). Pericytes regulate the blood-brain barrier. Nature 468, 557-561
5. Zhao, Z., Nelson, A.R., Betsholtz, C., and Zlokovic, B.V. (2015). Establishment and Dysfunction of the Blood-Brain Barrier. Cell 163, 1064-1078
6. Braniste, V., Al-Asmakh, M., Kowal, C., Anuar, F., Abbaspour, A., Toth, M., Korecka, A., Bakocevic, N., Ng, L.G., Kundu, P., et al. (2014). The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 6, 263ra158
7. Radu, M., and Chernoff, J. (2013). An in vivo assay to test blood vessel permeability. J Vis Exp, e50062
8. Ku, M.C., Waiczies, S., Niendorf, T., and Pohlmann, A. (2018). Assessment of Blood Brain Barrier Leakage with Gadolinium-Enhanced MRI. Methods Mol Biol 1718, 395-408
Figures