0143
Breast tumour response to PEGPH20-induced stromal modulation assessed by multiparametric MRI
Emma L. Reeves1, Jin Li1, Jessica K. R. Boult1, Barbara Blouw2, David Kang2, Jeffrey C. Bamber1, Yann Jamin1, and Simon P. Robinson1
1Radiotherapy and Imaging, Institute of Cancer Research, London, United Kingdom, 2Halozyme Therapeutics, San Diego, CA, United States
1Radiotherapy and Imaging, Institute of Cancer Research, London, United Kingdom, 2Halozyme Therapeutics, San Diego, CA, United States
Synopsis
Degradation of hyaluronan by PEGPH20 can improve stromal-dense tumour response to therapy. Given PEGPH20 treatment is associated with a reduction in tumour water content, we hypothesised that T1, T2, MTR and ADC may inform on PEGPH20 response. MRI was performed before and after PEGPH20 treatment in 4T1 HAS3 and MDA-MB-231 LM2-4 orthotopic breast tumours. T1, T2, and ADC significantly decreased, and MTR significantly increased following PEGPH20 treatment in 4T1 HAS3 tumours. PEGPH20 significantly decreased ADC but did not change T1, T2 or MTR in MDA-MB-231 LM2-4 tumours. These data suggest that ADC can detect breast tumour response to PEGPH20.
Introduction
Breast cancer is characterised by a strong desmoplastic reaction resulting in extracellular matrix (ECM) rigidity. This stromal-dense phenotype can promote tumour progression and metastasis through aberrant tensional homeostasis, increased solid stress and elevated interstitial fluid pressure (IFP). Consequently, stromal modulating therapies and accompanying biomarkers are being developed to target ECM stiffness for therapeutic gain. PEGPH20 is a PEGylated, recombinant, human hyaluronidase which can enzymatically degrade hyaluronan (HA), a principal component of the ECM. Treatment with PEGPH20 can reduce IFP and improve pre-clinical stromal-dense tumour responses to chemo-, radio- and immunotherapy1-3. PEGPH20 is currently being tested in a phase 1b/2 clinical trial for treatment of patients with HER2-negative, HA-high metastatic breast cancer (NCT02753595). Non-invasive imaging biomarkers that can inform on PEGPH20 response would help guide personalised adaptation of therapy.HA is a large, negatively charged, hygroscopic glycosaminoglycan and its degradation has been associated with a reduction in tumour water content4,5. These data suggest a reorganisation of water molecules within the tumour microenvironment following HA degradation and provide a rationale to evaluate T1, T2, magnetisation transfer ratio (MTR) and apparent diffusion coefficient (ADC) as potential biomarkers of response to PEGPH20.
Methods
All experiments were performed in accordance with the UK Animals (Scientific Procedures) Act 1986. Murine 4T1 breast tumours which overexpress hyaluronan synthase 3 (HAS3) were propagated orthotopically in female BALB/c mice (n = 24) and human luc-MDA-MB-231 LM2-4 breast tumours were propagated orthotopically in female athymic NCr-Foxn1nu mice (n = 12). 4T1 HAS3 and MDA-MB-231 LM2-4 tumours (335 ± 23 mm3 and 456 ± 38 mm3 respectively) were imaged prior to and 24 hours after treatment with either saline or 1 mg/kg PEGPH20 (Halozyme Therapeutics).MRI was performed on a 7T Biospec 70/20 USR horizontal MRI system (Bruker Instruments, Ettlingen, Germany). MRI included the acquisition of anatomical T2-weighted images, inversion recovery (IR)-TrueFISP images (TE = 1.7 ms, TR = 3.4 ms) for estimating T1 and T2 relaxation times, MT-RARE (TE = 19.22 ms, TR = 1500 ms, frequency offset = 25 ppm ‘on’ and 100 ppm ‘off’) to derive the MTR, and diffusion-weighted MRI (DWI; TE = 37.88 ms, TR = 1500 ms) for determination of the ADC. Parametric maps of T1, T2, MTR and ADC were constructed from a 1 mm thick axial slice over a 3x3 cm FOV taken from the tumour centre. Median T1, T2, MTR, and ADC values were calculated from a region of interest (ROI) which contained all viable tumour tissue and excluded necrosis. MRI-aligned serial tissue sections (5 µm) were cut and stained with H&E for the assessment of morphology and necrosis. HA was detected by a biotinylated, recombinant HA-binding protein6 (HTI-601, Halozyme Therapeutics).
Statistical analyses were performed using Prism 8 (GraphPad) and data are presented as mean ± SEM. Significant differences were identified using an unpaired Student’s t-test with a significance level of 5% unless otherwise stated.
Results
Orthotopic 4T1 HAS3 and MDA-MB-231 LM2-4 breast tumours showed HA accumulation which was distributed fairly evenly across viable tumour regions, and almost completely degraded 24 hours after PEGPH20 treatment (HTI-601 staining; Figure 1). PEGPH20 treated 4T1 HAS3 tumours had significantly higher MTR, and significantly reduced T1, T2, and ADC compared to saline control 4T1 HAS3 tumours. PEGPH20 treatment of MDA-MB-231 LM2-4 tumours significantly decreased ADC but did not significantly change T1, T2, or MTR (Figure 2). Parametric maps of T1, T2, MTR and ADC revealed that PEGPH20-induced changes occurred fairly homogeneously across viable tumour regions (Figure 3). Within-subject test-retest coefficients of variation, determined from the pre- and 24 hours post-saline data for both 4T1 HAS3 and MDA-MB-231 LM2-4 tumours, were 2.4%, 8.4%, 6.4%, and 11.3% for T1, T2, MTR and ADC respectively.Discussion
PEGPH20 treatment of 4T1 HAS3 breast tumours in vivo increased MTR and decreased T1, T2, and ADC across viable tumour regions. These data are consistent with HA degradation across the whole tumour leading to reorganisation of water molecules and loss of water from the tumour mass as reported in other pre-clinical tumour models4,5. MDA-MB-231 LM2-4 tumours treated in vivo with PEGPH20 showed a decrease in ADC but no significant change in T1, T2 or MTR. Whilst MDA-MB-231 LM2-4 tumours accumulate HA (>50% positivity using HTI-601 staining), this is to a lesser extent than in the 4T1 HAS3 tumours. This lower level of baseline HA expression in the MDA-MB-231 LM2-4 model suggests that degrading HA with PEGPH20 may induce reorganisation of fewer water molecules and a smaller decrease in tumour water content. ADC was able to detect this subtle response to PEGPH20 treatment in MDA-MB-231 LM2-4 tumours, perhaps because it is sensitive to both the presence and mobility of water molecules. Collectively, these data suggest that ADC is a sensitive biomarker of breast tumour response to PEGPH20.Conclusion
Response of HA-accumulating pre-clinical breast tumours to the HA degrading agent PEGPH20 can be detected in vivo by a reduction in ADC. This study provides a rationale to incorporate DWI into an imaging-embedded clinical trial to inform on human breast tumour response to PEGPH20.Acknowledgements
We acknowledge funding from the Cancer Research UK Centre at the ICR, and from the Cancer Research UK Imaging Centre at the ICR, funding from the Cancer Research UK grant C16412/A27725, and European Union’s Horizon 2020 research and innovation programme under grant agreement No 668039, in association with a Children with Cancer UK Research Fellowship.References
- Provenzano, P.P., et al., Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma.Cancer Cell, 2012. 21(3): p. 418-29.
- Bahn, J.D., et al., Abstract 2501: PEGylated human recombinant hyaluronidase (PEGPH20) enhances radiotherapy in hyaluronan-rich solid tumors.Cancer Research, 2011. 71(8 Supplement): p. 2501.
- Clift, R., et al., Remodeling the Tumor Microenvironment Sensitizes Breast Tumors to Anti-Programmed Death-Ligand 1 Immunotherapy.Cancer Research, 2019. 79(16): p. 4149.
- Thompson, C.B., et al., Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models.Mol Cancer Ther, 2010. 9(11): p. 3052-64.
- DuFort, Christopher C., et al., Interstitial Pressure in Pancreatic Ductal Adenocarcinoma Is Dominated by a Gel-Fluid Phase.Biophysical Journal, 2016. 110(9): p. 2106-2119.
- Jadin, L., et al., Characterization of a Novel Recombinant Hyaluronan Binding Protein for Tissue Hyaluronan Detection.Journal of Histochemistry & Cytochemistry, 2014. 62(9): p. 672-683.
Figures
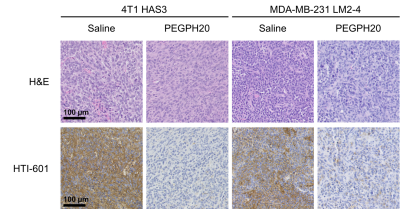
Figure 1. 4T1 HAS3 and MDA-MB-231 LM2-4 tumour tissue sections stained with H&E or HTI-601. Representative images (20x) are shown for one saline control and one PEGPH20 treated tumour per in vivo model. HTI-601 staining, which assesses hyaluronan (HA) accumulation, revealed different baseline HA levels in 4T1 HAS3 and MDA-MB-231 LM2-4 tumours and marked HA reduction in both models with PEGPH20 treatment.
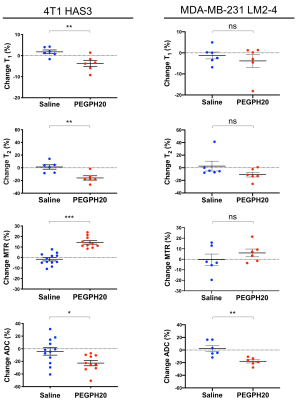
Figure 2. Percentage change in T1, T2, MTR, and ADC between pre- and post-treatment MRI for saline and PEGPH20 treated mice bearing 4T1 HAS3 or MDA-MB-231 LM2-4 tumours. PEGPH20 significantly reduced T1 (p=0.01), T2 (p=0.005), and ADC (p=0.02), and significantly increased MTR (p<0.0001) in 4T1 HAS3 tumours. PEGPH20 treatment of MDA-MB-231 LM2-4 tumours significantly decreased ADC (p=0.004) but no significant change was seen in T1 (p=0.94, Mann-Whitney), T2 (p=0.18, Mann-Whitney) or MTR (p=0.35).
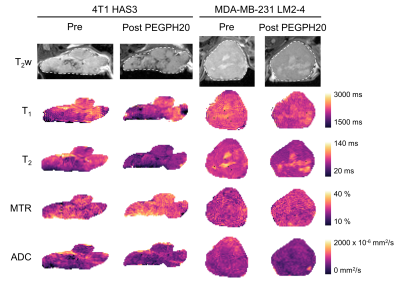
Figure 3. Anatomical T2-weighted MRI and parameter maps of T1, T2, MTR, and ADC, for one 4T1 HAS3 tumour and one MDA-MB-231 LM2-4 tumour prior to and 24 hours after PEGPH20 treatment (1mg/kg). The tumour ROI from which necrosis was excluded is shown by a white dashed line on the T2w images.