0141
Perfusion MRI related to survival, treatment response and sex differences in rectal cancer1Department of Oncology, Akershus University Hospital, Lørenskog, Norway, 2Department of Physics, University of Oslo, Oslo, Norway, 3Department of Diagnostic Physics, Division of Radiology and Nuclear Medicine, Oslo University Hospital, Oslo, Norway, 4Department of Radiology, Akershus University Hospital, Lørenskog, Norway, 5Institute of Clinical Medicine, University of Oslo, Oslo, Norway, 6Department of Physics, Norwegian University of Science and Technology, Trondheim, Norway
Synopsis
Three different MRI methods for obtaining perfusion related parameters were compared and evaluated as biomarkers in a study of 94 rectal cancer patients. The methods were dynamic contrast enhanced (DCE) MRI and dynamic susceptibility contrast (DSC) MRI analysed from a multi-echo dynamic EPI sequence, as well as intravoxel incoherent motion (IVIM) MRI analysed from a diffusion weighted sequence with 7 b-values. Tumour blood flow from DSC MRI was correlated to D* from IVIM MRI as well as Ktrans and vp from DCE MRI. Blood flow was also related to progression free survival, overall survival, treatment response and sex differences.
Introduction
Functional MRI, especially dynamic contrast enhanced (DCE) MRI, has shown some value for staging and treatment response evaluation in certain types of cancer. However, the results in rectal cancer have been highly heterogeneous (1). We examined perfusion related parameters from three functional MRI methods in the primary tumour of rectal cancer patients; DCE MRI, dynamic susceptibility contrast (DSC) MRI and intravoxel incoherent motion (IVIM) MRI, the relationship between them and their value as biomarkers.Methods
94 patients successfully underwent a multi-echo dynamic MRI with contrast agent injection and an extended diffusion weighted (DWI) sequence with seven b-values in addition to clinical procedure MRI. The scanning was done on a Philips Achieva 1.5 Tesla System (Philips Healthcare, Best, The Netherlands), with a five-channel cardiac coil. The dynamic data was obtained with a 3D multi-shot GRE EPI with three echoes (4.6, 13.9, 23.2 ms) with injection of a dose of 0.2 ml/kg body weight of a gadolinium-based contrast agent (Dotarem® 279.3 mg/ml, Guerbet Roissy, France). The flip angle was 28° and the repetition time was 39 ms. Time resolution varied between 1.9 and 2.5 s, and spatial resolution was 1.96 x 2.00 x 10 mm3. From the multi-echo data, the T1- and T2*-curves were extracted and used for an extended Tofts analysis (2) and a model-free deconvolution(3), respectively. This yielded the parameters Ktrans, kep, vp and ve from the DCE, and relative blood flow (BF) and area under curve (AUC) for 30 and 60 s from the DSC. Individual arterial input functions (AIFs) were extracted from the T2* data and applied for both analyses. The relative BF is related to the true perfusion, f, according to the equation:$$BF = \beta \, x\, f \frac{k_h}\rho$$
where kh is the haematocrit value, ρ the tissue density and β a scaling constant. To compare these values, it was therefore assumed that kh was the same for all patients, ρ the same for all tumours and β the same for all examinations. It was also assumed that the leakage was negligible for the estimation of BF from the initial part of residual function after deconvolution. The DWI was obtained with an SE EPI with b-values 0, 25, 50, 100, 500, 1000 and 1300 s/mm2. The repetition time was 3000 ms, the echo time was 75 ms, and the spatial resolution was 2.0 x 2.67 x 4 mm3. DWI-data were fitted to a bi-exponential IVIM equation, yielding the parameters perfusion fraction f, pseudo-diffusion D* and diffusion D. Tumour delineations were done on T2 weighted images with DWI as extra guidance by two radiologists with 7 and 14 years of experience with abdominal MRI. Tumour regions were then semi-automatically co-registered to the parametrical images, and the median values were extracted. Correlation analysis was done with Spearman's rank correlation test. Survival analysis was done with a univariable Cox regression, where progression free survival was estimated from date of inclusion to local recurrence, metastatic disease or death for patients without metastasis at time of diagnosis. Differences in treatment response were examined with Student’s t-test for variables that were normally distributed and a Mann-Whitney U-test for variables that were not, this was determined by the Shapiro-Wilk test. The latter was done on the group of patients receiving neoadjuvant treatment (n = 43) where the resected specimen was examined by a pathologist and given a ypT score (pathologically assessed T stage after treatment).
Results
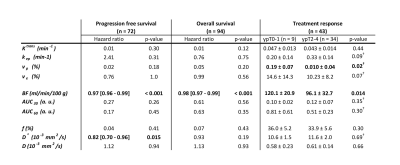
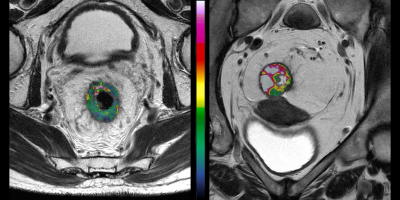
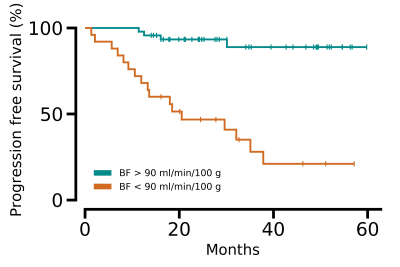
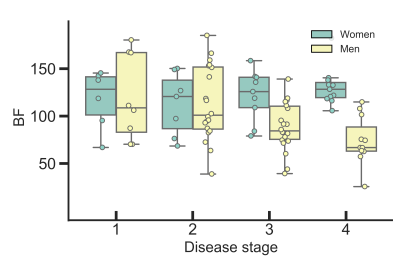
We found a correlation between BF and D* (rs = 0.47, p < 0.001), BF and Ktrans (rs = 0.29, p = 0.004), as well as BF and vp (rs = 0.44, p < 0.001). Low BF was associated with short progression free survival and overall survival for all patients, as well as poor treatment response for the patients receiving preoperative treatment before surgery, Table 1. Example images are given in Figure 1, and a Kaplan Meier plot for progression free survival is given in Figure 2. D* was also related to progression free survival and vp to treatment response. We also observed a sex difference in BF, at higher tumour stages, where women had higher BF than men (125 ± 27 ml/min/100 g for women versus 74 ± 26 ml/min/100 g for men at stage 4, p < 0.001), Figure 3.Discussion
This comparison of DSC, DCE and IVIM MRI in rectal cancer patients suggests that BF from DSC was the best biomarker for progression free survival, overall survival and treatment response. A low BF was related to poor outcome and may reflect poor oxygen delivery to the tissue and possibly hypoxia, a known marker of aggressive disease, metastatic development and poor treatment response. The assumptions for comparing the relative BF between patients, including the reproducibility of the AIF, are debatable, but our results suggest that they are within reason. DSC is not commonly used in extracranial cancers and these results supports further investigations of this method outside the brain.Conclusion
DSC should be further investigated as a method for obtaining biomarkers in extracranial cancers.Acknowledgements
No acknowledgement found.References
1. Dijkhoff RAP, Beets-Tan RGH, Lambregts DMJ, Beets GL, Maas M. Value of DCE-MRI for staging and response evaluation in rectal cancer: A systematic review. Eur J Radiol. 2017;95:155-68.
2. Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast‐enhanced t1‐weighted MRI of a diffusable tracer: Standardized quantities and symbols. J Magn Reson Imaging. 1999;10(3):223-32.
3. Østergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med. 1996;36(5):715-25.
Figures