0139
3D multi-shot diffusion imaging of the prostate with inter-shot correction and dictionary-based ADC matching1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Siemens Healthcare Limited, Frimley, United Kingdom, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Cancer Imaging Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Synopsis
Diffusion imaging is a key contrast in assessing prostate cancer. However, current single-shot EPI-based techniques are often distorted and fundamentally limited in resolution. The aim of this study is to develop multi-shot diffusion-prepared gradient echo imaging to obtain accurate 3D ADC maps in the prostate. We developed a 3D Cartesian centric trajectory with self-navigation, and a shot rejection approach to correct for inter-shot magnitude errors. We used a custom dictionary of acquisition specific signal evolutions to estimate ADC. We have shown in simulations and in vivo that accurate ADC values could be recovered.
Introduction
Diffusion imaging is a key contrast in prostate cancer imaging. Acquisition is typically based on single-shot EPI sequences with the aim to overcome the issue of motion corruption while encoding the microstructure contrast. However, resulting images are often distorted and fundamentally limited in resolution. There is a clear clinical need to improve the performance of this key cancer imaging contrast to be used in precision diagnostics and treatment guidance. Multi-shot diffusion-prepared sequences can be combined with 3D k-space trajectories to achieve non-distorted and high resolution diffusion images. However, technical challenges are that: 1) motion leads to intershot signal magnitude errors (MiMe) 1-3; and 2) a simple exponential model to estimate the ADC is not applicable. Both, 1) and 2) can lead to ADC quantification errors. The aim of this study is to address these two challenges to obtain accurate 3D ADC maps in the prostate.Methods
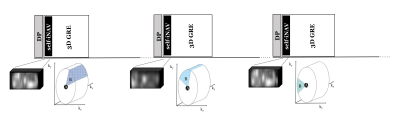
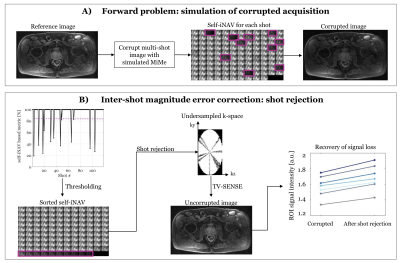
In this study we used a prototype 3D multi-shot diffusion-prepared sequence with gradient echo readout (MS-DP-GRE), with a twice refocused spin echo module and bipolar diffusion gradients 4. All data were acquired on a 3T PET-MR scanner (Biograph mMR, Siemens Healthcare, Erlangen, Germany).To address challenge 1) of inter-shot errors, we developed a custom 3D Cartesian centric trajectory (Figure 1) with self-navigation, and a shot rejection approach to correct for inter-shot magnitude errors. Specifically, from the self-image navigators (self-iNAVs) we calculate an image intensity-based correlation metric to detect and discard corrupted shots. We then reconstruct the remaining undersampled k-space data using total variation SENSE (TV-SENSE) 5. Details of the approach are described in Figure 2.
Experiments challenge 1. We first tested the approach in a simulation corrupting an image with simulated MiMe. We then compared the signal intensity of the corrected image with the original uncorrupted one for different prostate regions of interest (ROIs). Next, we tested the shot rejection approach in-vivo using MS-DP-GRE and the following imaging parameters: 78 RF pulses per shot (of which 10 used for self-navigation), shot duration (TR) = 1000 ms, flip angle = 10o, TR/TE = 5.0/2.5 ms, 400 Hz/px bandwidth, transversal orientation, 256x168x30 matrix, 1.6x1.6x5 mm3 resolution, DP-TE = 90 ms, b-value = 50, 400 s/mm2, diffusion direction = slice, phase, and read, acquisition time per volume = 1:57 min.
To address challenge 2) we used EPG simulations 6 to model the exact acquisition-specific diffusion signal evolution and to generate a dictionary of simulated signals for different tissue types T1/T2 = 1700/100 ms, ADC = [0.4, …, 2.8] μm2/ms. The ADC maps are generated via voxel wise matching between measured diffusion signal and the simulated dictionary, similar to 7.
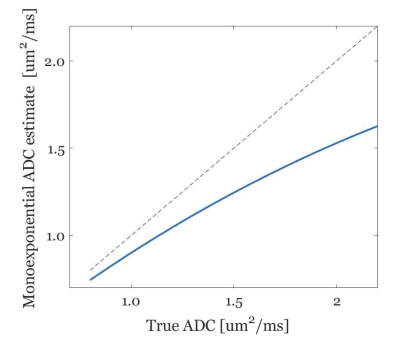
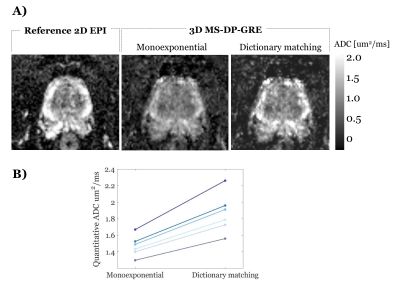
Experiments challenge 2. To illustrate the bias of the monoexponential fitting, we estimated the ADC values using the monoexponential fitting applied to the MS-DP-GRE simulated signal. To test feasibility of the proposed dictionary-based ADC mapping in the prostate, for an exemplar prostate diffusion dataset (b-values = 50,800 s/mm2, other imaging parameters as above) the ADC map was obtained with both standard monoexponential fitting and the proposed dictionary-matching.
Results and Discussion
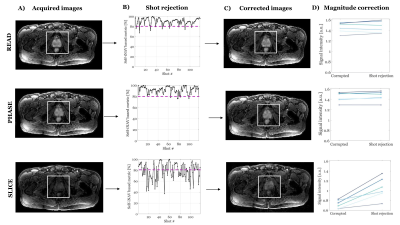
Figure 2B shows the ability of the proposed shot-rejection approach to recover signal loss due to simulated MiMe. Figure 3 shows an in-vivo case. In the read and phase diffusion directions only minor corruptions were observed, whilst in the slice direction there were significant signal dropouts due to intershot motion (Figure 3A), as also shown by the self-iNAV based metric (Figure 3B). A threshold of 80% in the similarity metric led to a 14% and 4% shot rejection in read and phase, and 50% in the slice direction. After shot rejection and TV-SENSE reconstruction the signal could be recovered.Figure 4 illustrates the underestimation of ADC values when using the monoexponential fitting for a representative prostate tissue type (T1 = 1700ms, T2 = 100ms). Also in vivo using the monoexponential fitting the ADC values were underestimated compared to the reference EPI-ADC. Using the dictionary-based approach the ADC values increased matching more closely the EPI-ADC reference values (Figure 5).
Conclusion
The proposed shot rejection approach was able to recover signal magnitude loss due to motion-induced intershot magnitude errors both in simulations and in-vivo. The dictionary matching approach allowed a more accurate estimation of ADC values compared to the standard monoexponential fitting.Acknowledgements
This work was supported by the King’s College London & Imperial College London EPSRC Centre for Doctoral Training in Medical Imaging [EP/L015226/1]; the Wellcome EPSRC Centre for Medical Engineering at Kings College London [WT 203148/Z/16/Z]; the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London; the King’s Health Partners Research and Development Challenge Fund; TOHETI; NIHR BRC; GSTT/KCL BRC; CRUK/EPSRC Cancer Centre; Siemens Healthineers. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
1. Cervantes B, Van AT, Weidlich D, Kooijman H, Hock A, Rummeny EJ, et al. Isotropic resolution diffusion tensor imaging of lumbosacral and sciatic nerves using a phase-corrected diffusion-prepared 3D turbo spin echo. Magn Reson Med. 2018;80: 609–618.
2. Gao Y, Han F, Zhou Z, Zhong X, Bi X, Neylon J, et al. Multishot diffusion-prepared magnitude-stabilized balanced steady-state free precession sequence for distortion-free diffusion imaging. Magn Reson Med. 2018; 1–11.
3. Zhang Q, Coolen BF, Nederveen AJ, Strijkers GJ. 3D Diffusion Imaging with SPiral Encoded Navigators from Stimulated Echoes (3D DISPENSE). Magn Reson Med. 2018; 1–14.
4. Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49: 177–182.
5. Cruz G, Atkinson D, Buerger C, Schaeffter T, Prieto C. Accelerated motion corrected three-dimensional abdominal MRI using total variation regularized SENSE reconstruction. Magn Reson Med. 2016;75: 1484–1498.
6. Weigel M. Extended phase graphs: Dephasing, RF pulses, and echoes - Pure and simple. J Magn Reson Imaging. 2015;41: 266–295.
7. Roccia E, Vidya Shankar R, Neji R, Cruz G, Munoz C, Botnar R, et al. Accelerated 3D T2 mapping with dictionary-based matching for prostate imaging. Magn Reson Med. 2018;81: 1795–1805.
Figures