0128
Metabolic modulation towards improved outcome in human glioblastoma model1Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Radiation Oncology, University of Pennsylvania, Philadelphia, PA, United States, 3Radiation Oncology, Thomas Jefferson University, Philadelphia, PA, United States, 4Neurosurgery, University of Alabama, Birmingham, AL, United States, 5Radiation Oncology, University of Iowa, Iowa City, IA, United States, 6Biostatistics and Epidemiology, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Standard of care for glioblastoma multiforme (GBM) patients, the Stupp protocol, involves radiotherapy concurrent with adjuvant temozolomide (TMZ) chemotherapy. Lonidamine (LND), an inhibitor of monocarboxylate transporters, mitochondrial pyruvate carrier and mitochondrial complex I & II, is shown to potentiate TMZ chemotherapy inhibiting the growth of U251 glioblastoma cells orthotopically implanted in mice. LND effects measured in vivo by 31P and 1H MRS in subcutaneous U251 glioblastoma xenografts showed a sustained and tumor-selective decrease in intracellular pH, decrease in bioenergetics (βNTP/Pi) and an increase in lactate. Selective tumor acidification and deenergization induced by LND potentiated the TMZ response in U251 glioblastoma xenografts.
INTRODUCTION
The median overall survival of glioblastoma multiforme (GBM) patients has remained at 15 months since introduction of temzolomide (TMZ) in 2005.1 About 30% of GBM patients develop TMZ resistance within the first cycle of radiotherapy (RT) and TMZ treatment.2 Since TMZ and RT are the standard therapies for treatment of GBM patients,3 we propose to use lonidamine (LND) to potentiate TMZ chemotherapy. LND inhibits monocarboxylate transporters (MCTs), the mitochondrial pyruvate carrier (MPC) and mitochondrial complex I & II of the electron transport chain, inducing intracellular acidification, lactate accumulation and inhibition of ATP production.4-6 We anticipate that LND will selectively acidify GBM leading to inhibition of glutathione-S-transferase (GST)7 which will neutralize the diazomethane active free radical intermediate of TMZ8; tumor acidification will also inhibit methyl guanine-DNA methyltransferase (MGMT) activity that is critical to repair TMZ induced DNA damage.9 LND lowers tumor ATP levels4, 5 which is expected to decrease multidrug resistance. Furthermore, LND might overcome TMZ resistance by inhibiting mitochondrial metabolism that plays a critical role in TMZ resistance and production of reactive oxygen species.METHODS
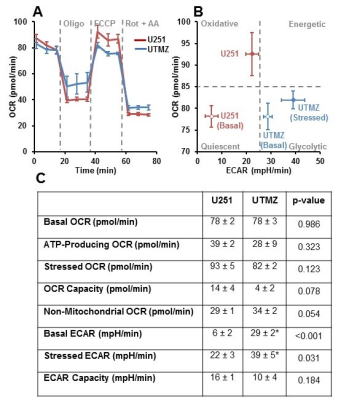
U251 (TMZ sensitive) and UTMZ (TMZ resistant) glioma cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, and 1% penicillin-streptomycin.In vitro oxygen consumption and extracellular acidification rates (OCR and ECAR) were determined using the Seahorse XF-96 Extracellular Flux Analyzer (Figure 1 caption).
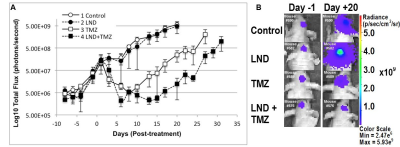
0.5×106 U251 cells transfected with firefly luciferase gene as a 2.5 µL suspension were injected stereotactically in brain of each mouse to develop intracranial tumors as described by Baumann et al.10 Four cohorts of 3-5 mice bearing orthotopic GBM tumors were used to assess the intracranial tumor growth delay after TMZ chemotherapy by bioluminescent imaging (BLI) (Figure 2 caption).
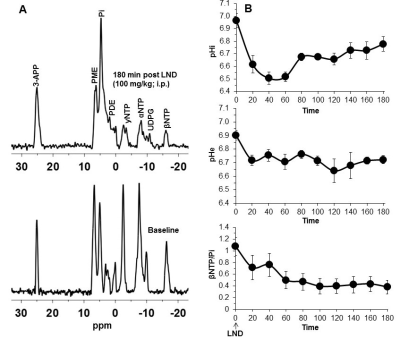
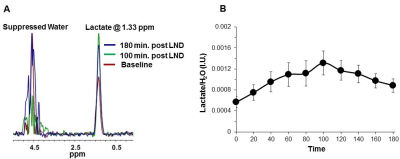
2×106 U251 cells were inoculated subcutaneously (s.c.) in each mouse (n=5) as a 0.1 mL suspension. 31P and 1H MRS experiments (n=5) were performed after positioning the s.c. tumor in a dual-frequency slotted-tube resonator; the intracellular pH (pHi), extracellular pH (pHe), bioenergetics (βNTP/Pi) (Figure 3 caption), and lactate levels were measured after LND (100 mg/kg; i.p.) administration using methods as described in our previous publication (Figure 4 caption).11, 12
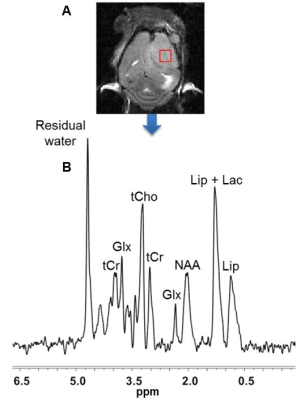
The 1H MRS spectrum of the intracranial tumor was measured utilizing the PRESS pulse sequence (Figure 5 caption).
One-way ANOVA and t-test analyses were performed for statistical considerations.
RESULTS
Although U251 has greater oxygenation capacity under stress, the two cell lines are very similar under basal oxygenation conditions. The glycolytic capacity of UTMZ is substantially greater than that of U251 (p < 0.001) under basal conditions, but under stress the two lines become more similar (p = 0.031) (Figure 1 and table 1). We monitored intracranial tumor growth by BLI after TMZ chemotherapy. Figure 2 shows that TMZ produced a growth delay of ~15 days, whereas pretreatment with LND extends the growth delay of TMZ by ~50% to ~23 days. Figure 3A shows representative localized 31P MR spectra of subcutaneous U251 glioma xenografts before and after LND treatment. Figure 3B shows changes in pHi, pHe, bioenergetics and lactate in response to LND in subcutaneous U251 xenografts. LND produces a sustained and tumor-selective decrease in pHi from 6.95 ± 0.06 to 6.51 ± 0.04 (p < 0.001), and pHe from 6.91 ± 0.03 to 6.71 ± 0.03 (p > 0.05). Tumor bioenergetics (βNTP/Pi) decreased by 73.0 ± 0.09% (p = 0.01) relative to the baseline level immediately prior to LND administration. Figure 4A shows steady-state levels of tumor lactate monitored by 1H MRS with the HDMD-SelMQC transfer pulse sequence in U251 glioma xenografts following LND administration. The lactate intensity continuously increased until 100 min and then decreased monotonically (Figures 4A & B). Figure 5 shows the 1H MRS spectrum of the intracranial tumor (PRESS pulse sequence). The lactate peak overlaps an intense lipid peak at 1.3 ppm.DISCUSSION
We have shown that LND potentiates the activity of a number of N-mustards,13, 14 which results in part from LND-mediated acid inhibition of MGMT,9 an enzyme that repairs DNA damage caused by alkylation of guanine in runs of multiple G residues. This leads to mispairing of alkylated-G with T instead of C residues of DNA. In addition, the active intermediate for N-mustards, the aziridinium cation,15 is stabilized by acid since OH- can neutralize this reactive intermediate, which is irreversibly deactivated by GST, an enzyme that is also inhibited by acid.7 We now propose that the same mechanism applies to TMZ; here the active alkylating intermediate, diazomethane,8 is also subject to stabilization by H+ and deactivation by GST. In the case of TMZ, methylation of DNA again occurs at nucleophilic runs of consecutive G residues and is also repaired by MGMT, which we propose will also be inhibited by LND-induced selective tumor acidification.11, 16 There remains a pressing need for noninvasively detected biomarkers quantification of active and inactive MGMT and development of methods to overcome TMZ resistance by delineating the metabolic basis of resistance.CONCLUSION
Our results suggest possible treatment protocol changes for improved outcomes in GBM patients receiving TMZ. This result from LND induced changes that enhance both LND and TMZ activities.Acknowledgements
Bridging fund, Perelman School of Medicine, University of PennsylvaniaReferences
1. Payne MJ, Pratap SE & Middleton MR. Temozolomide in the treatment of solid tumours: current results and rationale for dosing/scheduling. Crit Rev Oncol Hematol. 2005;53(3):241-52.
2. Griguer CE, Cantor AB, Fathallah-Shaykh HM, et al. Prognostic relevance of cytochrome C oxidase in primary glioblastoma multiforme. PLoS One. 2013;8(4):e61035. PMCID: 3622606.
3. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-66.
4. Guo L, Shestov AA, Worth AJ, et al. Inhibition of mitochondrial complex II by the anti-cancer agent lonidamine. J Biol Chem. 2016;291(1):42-57.
5. Nancolas B, Guo L, Zhou R, et al. The anti-tumour agent lonidamine is a potent inhibitor of the mitochondrial pyruvate carrier and plasma membrane monocarboxylate transporters. Biochem J. 2016.
6. Cheng G, Zhang Q, Pan J, et al. Targeting lonidamine to mitochondria mitigates lung tumorigenesis and brain metastasis. Nat Commun. 2019;10(1):2205. PMCID: 6525201.
7. Board PG, Taylor MC, Coggan M, et al. Clarification of the role of key active site residues of glutathione transferase zeta/maleylacetoacetate isomerase by a new spectrophotometric technique. Biochem J. 2003;374(Pt 3):731-7. PMCID: 1223650.
8. Denny BJ, Wheelhouse RT, Stevens MF, et al. NMR and molecular modeling investigation of the mechanism of activation of the antitumor drug temozolomide and its interaction with DNA. Biochemistry. 1994;33(31):9045-51.
9. Pegg AE, Wiest L, Foote RS, et al. Purification and properties of O6-methylguanine-DNA transmethylase from rat liver. J Biol Chem. 1983;258(4):2327-33.
10. Baumann BC, Dorsey JF, Benci JL, et al. Stereotactic intracranial implantation and in vivo bioluminescent imaging of tumor xenografts in a mouse model system of glioblastoma multiforme. J Vis Exp. 2012(67). PMCID: 3490274.
11. Nath K, Nelson DS, Ho AM, et al. 31P and 1H MRS of DB-1 melanoma xenografts: lonidamine selectively decreases tumor intracellular pH and energy status and sensitizes tumors to melphalan. NMR Biomed. 2013;26(1):98-105. PMCID: 3465621.
12. Nath K, Roman J, Guo L, et al, editor. Effect of lonidamine on response of human glioma model response to temozolomide. Proc Intl Soc Magn Reson Med (ISMRM) 2018; Paris.
13. Nath K, Nelson DS, Putt ME, et al. Comparison of the Lonidamine Potentiated Effect of Nitrogen Mustard Alkylating Agents on the Systemic Treatment of DB-1 Human Melanoma Xenografts in Mice. PLoS One. 2016;11(6):e0157125.
14. Nath K, Nelson DS, Roman J, et al. Effect of Lonidamine on Systemic Therapy of DB-1 Human Melanoma Xenografts with Temozolomide. Anticancer Res. 2017;37(7):3413-21.
15. Kohn KW, Hartley JA & Mattes WB. Mechanisms of DNA sequence selective alkylation of guanine-N7 positions by nitrogen mustards. Biochem Pharmacol. 1988;37(9):1799-800.
16. Nath K, Nelson DS, Heitjan DF, et al. Lonidamine induces intracellular tumor acidification and ATP depletion in breast, prostate and ovarian cancer xenografts and potentiates response to doxorubicin. NMR Biomed. 2015;28(3):281-90.
Figures