0125
The immune checkpoint PD-L1 alters choline kinase expression and metabolism in triple negative breast cancer cells1The Russell H Morgan Department of Radiology and Radiological Science, The Johns Hopkins University, School of Medicine, Baltimore, MD, United States, 2Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins University, School of Medicine, Baltimore, MD, United States, 3Radiation Oncology and Molecular Radiation Sciences, The Johns Hopkins University, School of Medicine, Baltimore, MD, United States
Synopsis
Expression of programmed death-ligand 1 (PD-L1) plays a significant role in creating an immune suppressive tumor microenvironment. We investigated the relationship between the aberrant choline metabolism observed in most cancers and PD-L1 expression in triple negative human MDA-MB-231 breast cancer cells. Using siRNA to downregulate Chk-a or PD-L1 or both, we identified a close inverse interdependence between Chk-α and PD-L1. We identified, for the first time, the role of PD-L1 in cancer cell metabolism. These results have significant implications for therapy and provide new insights into the relationship between metabolism and immune resistance in these breast cancer cells.
Introduction
Cancer immunotherapy is designed to activate the immune system against cancer cells. The field has been revitalized with the discovery of immune checkpoints such as programmed cell death protein-1 (PD-1) and its ligand (PD-L1, CD274)1. As the regulators of the immune system activation, these immune checkpoints are utilized by cancer cells to escape immune surveillance. Once PD-L1 binds to PD-1, the T cell becomes deactivated inhibiting the natural immune response to cancer. Immune checkpoint inhibitor therapies, such as the use of anti-PD-1 or PD-L1 monoclonal antibodies, trigger an immune response against cancer cells by blocking immune checkpoints used by cancer cells to escape immune destruction. PD-L1 is often overexpressed in cancers and it has been successfully exploited for immune therapy2 in different tumor types, such as melanoma, non-small cell lung carcinoma and renal cell carcinoma3,4. However, treatment outcomes have not been as effective in other cancers such as breast5 and pancreatic cancer6. Although the explanation for this is likely multifactorial, different studies point towards cancer metabolism as one of the possible causes7-9.One of the most common tumor associated metabolic alteration is aberrant choline (Cho) metabolism, characterized by increased phosphocholine (PC) and total choline-containing compounds (tCho). Increased PC detected as an increase of total choline by 1H Magnetic Resonance Spectroscopy (MRS) in most cancers is mainly due to increased expression of choline kinase-α (Chk-α). Since the presence of PC on proteins has been related to escape from immune surveillance10, here, we investigated the relationship between Chk-α, PC and PD-L1 in triple negative MDA-MB-231 human breast cancer cells.
Materials and methods
Experiments were performed using triple negative breast MDA-MB-231 cells that were transiently transfected with small interfering RNA (siRNA) against either luciferase (used as control siRNA), Chk-α or PD-L1 following standard protocols. Total RNA was isolated, complementary cDNA synthesised and quantitative real-time PCR (q-RT-PCR) performed using IQ SYBR Green supermix and gene specific primers following an established protocol11.For high resolution 1H MRS, cell extracts were obtained using a dual-phase extraction method as previously described11. Water-soluble samples were dissolved in 0.6 mL of buffered D2O (Sigma). Lipids-phase samples were dissolved in 0.6 ml of a mixture of CDCl3:MeOD in a 2:1 ratio. High-resolution 1H MR spectra were recorded on a Bruker Biospin Avance-III 750 MHz NMR (Bruker Biospin) spectrometer operating at a proton frequency of 750.21 MHz using a 5-mm broad band inverse (BBI) probe head equipped with z-gradient accessories. 1H MR spectra were manually phased and baseline corrected using TOPSPIN 3.2 software. Integrals of the metabolites of interest were determined and normalized to the TSP reference and the number of cells. Metabolites were estimated from at least three experimental samples. Statistical significance was evaluated using the Student t-test.
Results and Discussion
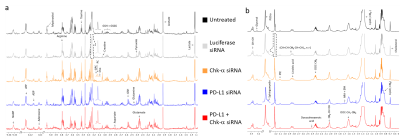
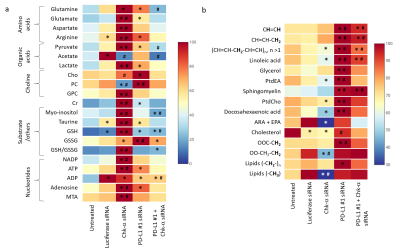
We observed that silencing Chk-α resulted in a significant increase of PD-L1 expression and silencing PD-L1 resulted in a significant increase of Chk-α expression12. This inverse relationship was eliminated when both PD-L1 and Chk-α were silenced. Transfection with control siRNA did not affect Chk-α mRNA levels, but induced a small increase of PD-L1 compared to untreated cells.To demonstrate that changes in Chk-α mRNA following PD-L1 down regulation translated to functional metabolic changes, we performed high resolution 1H MRS of cell extracts to measure metabolites in the water soluble and lipid phases, as shown in the representative spectra in Figure 1. Consistent with the molecular changes, a significant increase of PC was observed in cells transfected with PD-L1 siRNA compared to cells transfected with control siRNA (Figure 2a). Metabolic changes, however, extended well beyond the choline containing compounds. We identified changes in amino acids, organic acids, nucleotides and other compounds such as myo-inositol, taurine and glutathione (Figure 2a). Furthermore, we found that PD-L1 downregulation profoundly altered the lipid profile of MDA-MB-231 cells, increasing the total amount of lipids, the total amount of unsaturated lipids as well as individual lipids such as linoleic acid, glycerol, sphingomyelin, docosahexaenoic acid, and cholesterol (Figure 2b).
These data suggest that treatments that decrease Chk-α could result in cancer cells escaping immune surveillance through increased expression of PD-L1. Metabolic characterization showed that cells with downregulated Chk-α levels shifted towards a more immunosuppressive profile through metabolic reprogramming, increasing the production of metabolites that have been linked to decreased effectiveness of T-cells13. Our data also suggest that low levels of PD-L1 can skew cancer cell metabolism towards a more immunosuppressive profile by a significant increase of lipid production and changes in the lipid profile14. These results provide new insights in the role of PD-L1 in the cancer metabolome that may be exploited to improve the outcome of treatment with immune checkpoint inhibitors.
Acknowledgements
This work was supported by NIH R35CA209960 and R01CA82337. JPT was funded by Fundación Alonso Martín Escudero and MSCA.References
1. Keir ME, Francisco LM, and Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007; 19(3):309-14.
2. Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014; 2(4):361-70.
3. Tang J, Yu JX, Hubbard-Lucey VM, et al. Trial watch: The clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat Rev Drug Discov. 2018; 17(12):854-855.
4. Prat A, Navarro A, Pare L, et al. Immune-Related Gene Expression Profiling After PD-1 Blockade in Non-Small Cell Lung Carcinoma, Head and Neck Squamous Cell Carcinoma, and Melanoma. Cancer Res. 2017; 77(13):3540-3550.
5. Tan TJ, Chan JJ, Kamis S, et al. What is the role of immunotherapy in breast cancer? Chin Clin Oncol. 2018; 7(2):13.
6. Aroldi F and Zaniboni A. Immunotherapy for pancreatic cancer: present and future. Immunotherapy. 2017; 9(7):607-616.
7. Li X, Wenes M, Romero P, et al. Navigating metabolic pathways to enhance antitumour immunity and immunotherapy. Nat Rev Clin Oncol. 2019.
8. Singer K, Cheng WC, Kreutz M, et al. Immunometabolism in cancer at a glance. Dis Model Mech. 2018; 11(8).
9. Wang X, Ping FF, Bakht S, et al. Immunometabolism features of metabolic deregulation and cancer. J Cell Mol Med. 2019; 23(2):694-701.
10. Lovell TM, Woods RJ, Butlin DJ, et al. Identification of a novel mammalian post-translational modification, phosphocholine, on placental secretory polypeptides. J Mol Endocrinol. 2007; 39(3):189-98.
11. Penet MF, Shah T, Bharti S, et al. Metabolic imaging of pancreatic ductal adenocarcinoma detects altered choline metabolism. Clin Cancer Res. 2015; 21(2):386-95.
12. Pacheco-Torres J, Penet MF, Mironchik Y, Krishnamachary B, Bhujwalla ZM. The Immune Checkpoint PD-L1 and Choline Kinase-α are inversely related in triple negativehuman breast cancer cells. ISMRM 2018, Abstract # 1533
13. Xia X, Zhou W, Guo C, et al. Glutaminolysis Mediated by MALT1 Protease Activity Facilitates PD-L1 Expression on ABC-DLBCL Cells and Contributes to Their Immune Evasion. Front Oncol. 2018; 8:632.
14. Herber DL, Cao W, Nefedova Y, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010; 16(8):880-6.
Figures