Xiaojing Long1, Yangzi Qiao1, Teng Ma1, Weibao Qiu1, Chao Zou1, Jo Lee1, Yang Liu1, Changjun Tie1, Ye Li1, Lijuan Zhang1, Qiang He2, Xin Liu1, and Hairong Zheng1
1Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2Shanghai United Imaging Healthcare Co., Ltd., Shanghai, China
Synopsis
In
this work, we applied BOLD fMRI in Rhesus monkey on a 3T MR system and investigated the functional effects induced by transcranial ultrasound
stimulation (TUS) in both the target
spot (the primary visual
cortex) and the remote
interconnected brain regions. We found that TUS can evoke BOLD reaction not only on the region-specific
region but also the interconnected areas in the monkey brain. Additionally, our
results demonstrated that the temporal features of BOLD time courses of TUS on the primary visual
cortex and those of real visual stimulation have no significant difference in
the regions of primary visual pathway.
INTRODUCTION
Transcranial
ultrasound stimulation (TUS) is a noninvasive tool that can excite or inhibit
neural activity in targeted brain regions by delivering pulsed ultrasonic waves. The combination of
neuro-stimulation with functional MRI (fMRI) has received increasing attention
over the past years as fMRI could provide a good illustration of
brain responses to stimulation and their relation to cognition and perception,
as well as a better understanding of the mechanisms underlying modulation
effects. In this work, we applied BOLD fMRI in Rhesus monkeys on a 3T MR system, aiming to study the brain effects induced by
TUS in both the target
spot and the remote interconnected brain regions.METHODS
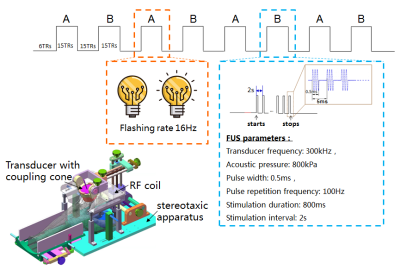
Experiments
were conducted on a 3T MR scanner (uMR790,
United Imaging Healthcare; Shanghai; China) with the simultaneous strength of 80
mT/m, slew rate of 200 mT/m/s, and a customized 8-channel surface coil. A customer-designed 300
kHz MR-compatible single element ultrasound transducer was used with a coupling cone filled with degassed ultrapure water, and fixed on the hair-removed
scalp above the V1 area of the Rhesus monkey. The location of TUS target was confirmed using
the MR-based acoustic radiation force imaging (ARFI). The stimulation
experiment comprised two
functional runs: (1) a visual stimulation and (2) a TUS run. The stimulation
consisted of 8 blocks, starting with 12 seconds baseline followed by 30-second
duration blocks of visual or TUS stimuli repeated for four times with 30-second
baseline intervals in between. The visual stimulation was performed to the left eye of the monkey with a flashing LED. As for TUS, an acoustic pressure
of 800kPa was used for stimulation. The whole-brain fMRI data was obtained using
a single-shot gradient echo planar imaging (EPI) sequence with parameters of
TR=2000ms, TE=30ms, flip angle 90°, 64×64 matrix, 32 slices (interleaved),
1.5×1.5×2.5 mm3
voxel size. All fMRI data analysis was
performed off-line using SPM12 with the typical processing
pipeline.RESULTS
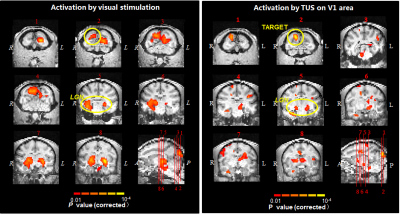
Activation
maps revealed that real visual stimulation activated primary visual cortex and
lateral geniculate nuclei (LGN) which are in the primary visual pathway, while
TUS not only activated the sonicated V1 area but also other brain regions including
LGN, anterior cingulate cortex (ACC), parahippocampal gyrus, supramarginal
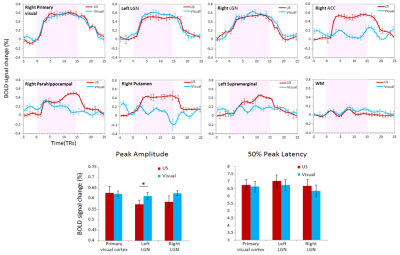
gyrus, caudate and putamen. By comparing the temporal features of the
event-related BOLD time courses, no significant difference was observed between
visual stimulation and TUS on the peak amplitude and 50% peak latency of BOLD
signals in the primary visual cortex and LGN.CONCLUSIONS
This
work have shown that TUS can serve as a novel non-invasive stimulation
technique to modulate region-specific and remote interconnected areas in the
monkey brain, which suggested that TUS may provide a new mode for
neuroscientific studies such as assessment of brain functions and their
functional connectivity to different parts of the brain. In addition, TUS may
also have the potentials for neurotherapeutics for remedying a certain
pathological conditions associated with regional or network dysfunction.Acknowledgements
This
work was supported by the National Natural Science Foundation of China
(No.81527901).References
No reference found.