0120
Active tracking based cardiac triggering of MR thermometry in an animal model1Biomedical Engineering, King's College London, London, United Kingdom, 2Siemens Healthcare GmbH, Erlangen, Germany, 3MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom, 4IHU-Liryc, Pessac, France, 5Imricor Medical Systems, Burnsville, MN, United States, 6Physikalisch-Technische Bundesanstalt, Berlin, Germany
Synopsis
MR thermometry can offer real-time temperature information during RF ablation in the heart. As ECG-triggering can be unreliable in these situations, cardiac triggering based on the position of the ablation catheter could provide an alternative. Active tracking was used to continuously measure the position of microcoils inside the catheter. Cardiac triggers were determined after respiratory motion filtering. Temperature stability over time was below 2.5 ˚C.
Introduction
MR thermometry can offer real-time feedback during MRI-guided catheter ablation to treat cardiac arrhythmias [1-5]. ECG-triggering can be negatively affected by RF ablation equipment and rapid gradient switching associated with the thermometry’s EPI-based acquisition [4]. Cardiac triggering based on continuous position determination of active tracking microcoils in the catheter (AT-triggering) has been suggested and demonstrated in phantom in the absence of respiratory motion [6]. This method is now expanded to filter out respiratory motion and was tested in-vivo in an animal model.Methods
Trigger determinationActive tracking (AT) modules (determining 3D position in 25 ms) were repeated until variations in their signal triggered the execution of single-shot MR-thermometry sequences. Trigger determination was implemented in the reconstruction software on the scanner. At the start of the sequence, at least 11 seconds of continuous AT signal were recorded to capture the AT signal over several breathing cycles. The following steps were then applied to all incoming AT measurements:
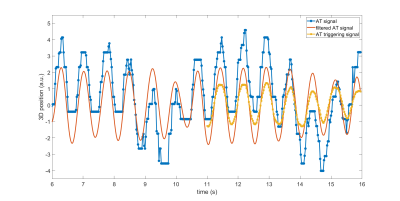
- For each new AT measurement at time t, a high-pass filter was applied to the last 10 seconds of AT signal to generate a respiratory filtered AT signal. The filtered signal at time t (i.e one time point) was stored as the entry of the AT triggering signal at time t (Figure 1).
- A trigger was detected when a peak was detected in the last 3 entries in the AT triggering signal and when the peak was within the highest 50 percentile, to avoid local maxima.
Thermometry with AT-based cardiac triggering (prototype) was performed within the left ventricle of a sedated and mechanically ventilated sheep (5 seconds breathing cycle). The stability of thermometry with AT-triggering was evaluated at two positions without RF energy deposition (100 dynamics), as well as during ablation (40W for 50 seconds, 150 dynamics). The experiments were conducted at 1.5 T (MAGNETOM Aera, Siemens Healthcare, Erlangen Germany) using an MR-compatible ablation catheter with active tracking microcoils in the tip (Vision-MR Ablation Catheter, Imricor Medical Systems, USA)). Proton resonance frequency shift based thermometry using EPI readout was performed as in [5], acquiring 4 slices per heartbeat. MR-thermometry reconstruction consisted of non-rigid motion correction, multi-baseline correction for respiratory-induced phase variation, as well as temporal filtering of temperature maps, as previously described in [7].
Results
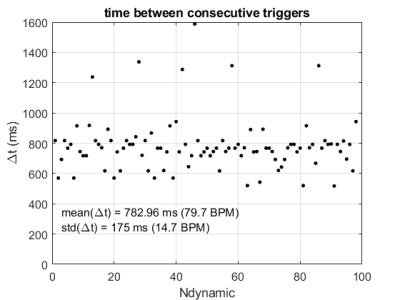
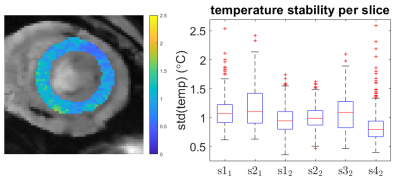
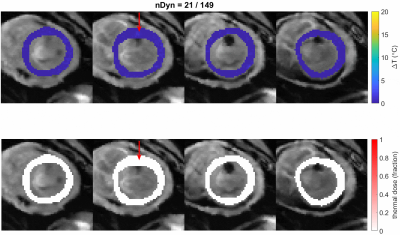
The time interval between the AT-based cardiac triggers is fairly stable and consistent with the heart rate (Figure 2), but outliers (>1200ms) indicate that some heart beats were missed. These mis-triggers were attributed to AT measurements in which there was insufficient SNR to determine the AT position correctly, causing occasional outlier AT signal positions. The stability of thermometry was calculated in 6 slices in which the myocardium was visible (Figure 3). Median values were below 1.5˚C and outliers within 2.5˚C. A localised elevation in temperature and associated lethal thermal dose could be observed during an ablation experiment (see Figure 4).Discussion
AT-based cardiac triggered MR-thermometry was successfully demonstrated during stability and ablation experiments. No ECG-triggering was used in these experiments and it may be insightful to compare ECG-triggering to AT-triggering in the future. AT could also be used for prospective respiratory motion correction (i.e. slice tracking), instead of cardiac triggering only, which will be the focus of future work.Conclusion
Active tracking was successfully used to perform cardiac triggering during MR-thermometry in an animal model.Acknowledgements
This work was supported by the EPSRC grant (EP/R010935/1) and the Health Innovation Challenge Fund (grant number HICF-R10-698), a parallel funding partnership between the Department of Health, and the Wellcome Trust. This work was also supported by the Wellcome EPSRC Centre for Medical Engineering at Kings College London (WT 203148/Z/16/Z) and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
1. Kolandaivelu, A. et al. Noninvasive assessment of tissue heating during cardiac radiofrequency ablation using MRI thermography. Circ. Arrhythmia Electrophysiol. 3, 521–529 (2010).
2. de Senneville, B. D. et al. Feasibility of fast MR-thermometry during cardiac radiofrequency ablation. NMR Biomed. 25, 556–562 (2012).
3. Ozenne, V. et al. Improved cardiac magnetic resonance thermometry and dosimetry for monitoring lesion formation during catheter ablation. Magn. Reson. Med. 77, 673–683 (2017).
4. Toupin, S. et al. Feasibility of real-time MR thermal dose mapping for predicting radiofrequency ablation outcome in the myocardium in vivo. J. Cardiovasc.Magn. Reson. 19, 14 (2017).
5. Mukherjee, R. K. et al. Epicardial electroanatomical mapping, radiofrequency ablation, and lesion imaging in the porcine left ventricle under real-time magnetic resonance imaging guidance—an in vivo feasibility study. EP Eur. (2017). doi:10.1093/europace/eux341
6. Mooiweer, R. et al. Active Tracking-based cardiac triggering of MR thermometry for MRI-guided cardiac ablation, ISMRM 2019
7 Roujol S, et al. Real-time MR-thermometry and dosimetry for interventional guidance on abdominal organs. Magn Reson Med 2010;63:1080–7.
Figures