0111
Feasibility of using transcranial magnetic stimulation devices to study magnetically induced cardiac stimulation in pigs1Computer Assisted Clinical Medicine, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany, 2A. A. Martinos Center for Biomedical Imaging, Department of Radiologoy, Massachusetts General Hospital, Charlestown, MA, United States, 3Harvard Medical School, Boston, MA, United States, 4Cardiovascular Research Center, Cardiology Division, Massachusetts General Hospital, Charlestown, MA, United States, 5Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, United States
Synopsis
This work evaluates the potential of porcine cardiac stimulation (CS) studies using transcranial magnetic stimulation (TMS) devices with the aim of determining appropriate safety limits for MRI gradients. We investigated the electric fields induced in electromagnetic porcine models and found that typical TMS coils may not generate fields strong enough for CS. Larger coplanar coils, however, may be suitable for CS studies. In addition to these investigations, we created a porcine model from MRI Dixon and cardiac CINE measurements. The use of such custom models of the animal under experimentation will facilitate the comparison between measured and simulated CS thresholds.
Purpose
Regulatory standards (e.g., IEC 60601-2-33) limit the switching speed dB/dt of MRI gradient systems to prevent peripheral nerve stimulation (PNS) and cardiac stimulation (CS)1. Unlike PNS, the CS limits cannot be safely measured in human volunteers. Therefore, the regulatory CS limits are based on electric field (E-field) thresholds derived from animal studies2,3, and simulations in simplified homogeneous human models3. The regulatory CS limits are conservative due to the severity of potentially induced cardiac arrhythmia4 and the limited data available. State-of-the-art gradient coils are increasingly restricted by these limits, suggesting the need for further study.We have extended our previous PNS modeling5,6 to study CS in human and animal models7. These simulations couple E-field calculations with electrophysiological models of cardiac Purkinje and ventricular muscle fibers to predict CS thresholds. Initial results showed a reasonable agreement between simulated and previously measured canine thresholds8,9,10. However, uncertainties remain about the exact experimental setup (e.g. animal anatomy, coil position), reducing its value for validation.
The porcine model has emerged as a primary animal model for the human cardiovascular system11. In this work, we evaluate the potential of porcine CS studies using two coils: 1) A transcranial magnetic stimulation (TMS) coil, and 2) a coplanar coil pair used in the previous canine studies8 to be driven by the TMS capacitor bank. We simulate the E-fields in the IT’IS porcine model12 (IT’IS foundation, Zurich, Switzerland) and compare them to the canine CS experiments and simulations to assess the likelihood of generating CS with these coils. Finally, we develop a porcine model from MRI images to facilitate future animal-specific CS analysis.
Methods
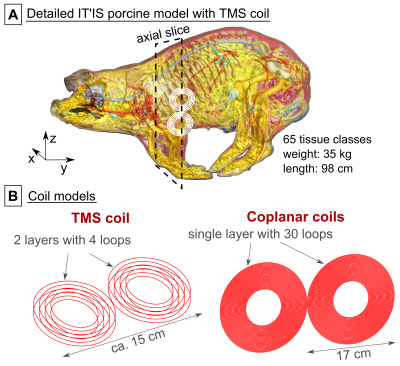
IT’IS model: We simulated the “full” (65 tissue classes) IT’IS porcine model12 (Fig. 1A) and a simplified version with only six tissue classes (soft tissue, heart, heart lumen, bone, air, skin).EM simulation: We modeled a typical TMS coil and the coplanar coil pair from the canine experiments8 (Fig. 1B) using a low-frequency quasi-static FEM solver (Sim4Life, Zurich MedTech, Switzerland) to calculate the E-fields for a sinusoidal coil current (1 kHz/1 A). We scaled the E-fields linearly using the respective coil efficiencies to match a dB/dt amplitude of 1000 T/s.
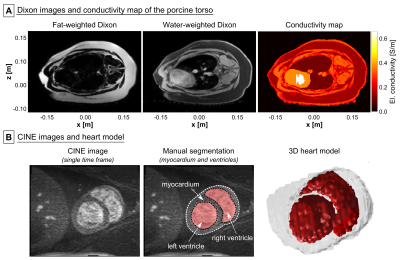
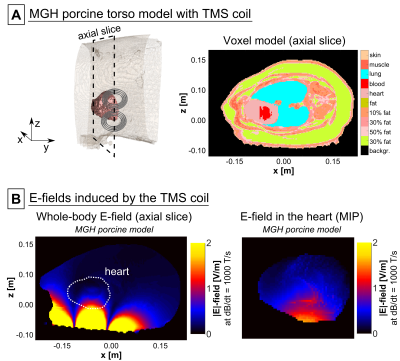
MGH model: MRI measurements of the torso of a pig were performed on the 3T MGH Connectome scanner (Siemens Healthcare, Erlangen, Germany). The Dixon method was used to acquire in-phase and out-of-phase volumes (TE/TR=2.45 ms/5.00 ms, resolution=1.4x1.4x1.3 mm), which were used to estimate the fat/water content SFW in each voxel (Fig. 2A). We assigned conductivity values of σ=SFW×σF+(1-SFW)×σM, where σF=0.057 S/m (fat) and σM=0.355 S/m (muscle), and grouped the voxels into six conductivity classes. We manually segmented the lung (σ=0.11 S/m), and assigned the outermost voxel layer as skin (σ=0.17 S/m). Finally, we used a CINE acquisition of the beating heart (TE/TR=2.34 ms/38.4 ms, resolution=1.4x1.4x1.5 mm) to segment the porcine heart (Fig. 2B).
Results
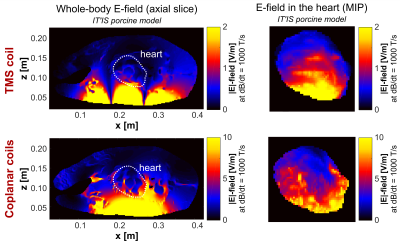
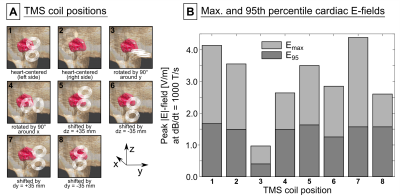
Figure 3 (top row) shows the E-field induced in the IT’IS model with the TMS coil placed on the left side of the torso. In the full IT’IS model, the maximum |E|-field in the heart was Emax=4.1 V/m (95th percentile |E|-field E95=1.7 V/m). In the simplified IT’IS model, Emax decreased by 7%, and E95 increased by 1%. The coplanar coils induce significantly higher E-fields than the TMS coil (Emax=11.8 V/m, E95=6.9 V/m, Fig. 3 bottom row). Additionally, we evaluated the E-fields in the heart of the IT’IS model for different positions of the TMS coil (Fig. 4). Figure 5 shows the E-field induced in the preliminary MGH model by the TMS coil (Emax=1.6 V/m, E95=1.0 V/m).Discussion
We recently studied magnetically induced CS in canines10. Our simulations and previous studies13 indicate that an average E95≈100 V/m is required to cause CS for a damped sinusoidal current of ~500 µs duration. Even with ideal coil positioning, generation of a similar E-field in the pig would require a peak dB/dt≈60 kT/s for the TMS coil (the E-field scales linearly with dB/dt). Typical commercial TMS devices reach a peak dB/dt≈40 kT/s, suggesting that these devices may not be powerful enough to generate CS with typical TMS coils. The larger coplanar coils, however, would require dB/dt≈15 kT/s to achieve E95≈100 V/m in the porcine heart, which is in agreement with the CS thresholds measured in canines8,10. As such, these coils may also be suitable to study CS in pigs.Simplifying the IT’IS porcine model only mildly affected the E-field in the heart, suggesting that body models with only few tissue classes may be adequate to predict the E-fields. We have tentatively demonstrated the creation of a custom porcine model using MRI Dixon and cardiac CINE data. The lower E-field in this model (compared to the IT’IS model) most likely results from truncation effects due to the limited torso extent (we will resolve this by extending the imaging FOV). The custom porcine models will eventually be augmented by our cardiac fiber models (which are not included in the IT’IS model), allowing a direct comparison of experimental and simulated CS thresholds. Such an integrative approach of measurements and simulations in animal (and human) models might play an important role in determining adequate safety limits for MRI gradients.
Acknowledgements
No acknowledgement found.References
[1] International Electrochemical Commission (IEC), “International standard IEC 60601-2-33 medical electrical equipment. Particular requirements for the safety of magnetic resonance equipment for medical diagnosis”. Technical Report 2010.
[2] Reilly JP. Magnetic field excitation of peripheral nerves and the heart: A comparison of thresholds. Med & Biol Eng & Comput. 1991;29:571-579.
[3] Irnich W. Electrostimulation by time-varying magnetic fields. MAGMA. 1994;2:43-49.
[4] Schaefer DJ, Bourland JD, Nyenhuis JA. Review of patient safety in time-varying gradient fields. JMRI. 2000;12:20-29.
[5] Davids M, Guérin B, vom Endt A, Schad LR, Wald LL. Prediction of peripheral nerve stimulation thresholds of MRI gradient coils using coupled electromagnetic and neurodynamic simulations. Magn Reson Med. 2019;81:686-701.
[6] Davids M, Guérin B, Malzacher M, Schad LR, Wald LL. Predicting magnetostimulation thresholds in the peripheral nervous system using realistic body models. Sci Rep. 2017;7:5316.
[7] Klein V, Davids M, Schad LR, Wald LL, Guérin B. Simulation of MRI gradient-induced cardiac stimulation: Are the current safety regulations too conservative? In: Proc Internat Soc Magn Reson Med. 2019; 729.
[8] Mouchawar GA, Bourland JD, Nyenhuis JA, Geddes LA, Foster KS, Jones JT, Graber GP. Closed-chest cardiac stimulation with a pulsed magnetic field. Med & Biol Eng & Comput. 1992;30:162-168.
[9] Nyenhuis JA, Bourland JD, Schaefer DJ, Foster KS, Schoelein WE, Mouchawar GA, Elabbady TZ, Geddes LA, Riehl ME. Measurement of cardiac stimulation thresholds for pulsed z-gradient fields in a 1.5-T magnet. In: Proc Soc Magn Reson Med. 1992;586.
[10] Klein V, Davids M, Schad LR, Wald LL, Guérin B. Simulation of electromagnetic cardiac stimulation: Validation in dogs and application to human threshold limits for MRI gradient coils. Submitted to Internat Soc Magn Reson Med. 2020; Abstract ID 1259.
[11] Dixon JA, Spinale FG. Large animal models of heart failure. Circ. 2009;2(3):262-271.
[12] Male pig v1.0, IT’IS Foundation. DOI: 10.13099/VIP91301-01-0.
[13] Ragan PM, Wang W, Eisenberg SR. Magnetically induced currents in the canine heart: A finite element study. IEEE Trans Biomed Eng. 1995;42(11):1110-1116.
Figures