0105
MRI assessment and monitoring of cavitation-based ultrasound therapy (histotripsy) for transcranial brain treatment in vivo1Biomedical Engineering, University of Michigan, Ann Arbor, MI, United States, 2Functional MRI Laboratory, University of Michigan, Ann Arbor, MI, United States, 3Department of Neurosurgery, University of Michigan, Ann Arbor, MI, United States
Synopsis
Transcranial MR guided cavitation-based Focused Ultrasound (FUS) treatment (histotripsy) is performed in vivo for the first time on a pig brain. Transcranial histotripsy is delivered by an MRI compatible FUS transducer array inside a 3T MRI scanner. Real-time MRI monitoring with 2 second temporal resolution is carried with an intra-voxel incoherent motion (IVIM) pulse sequence synchronized with the FUS array. IVIM images show the histotripsy ablation effect at the intended treatment location in real-time, and the ablation zone was confirmed by post-treatment images. This is the first study to show successful in vivo transcranial histotripsy guided by MRI.
INTRODUCTION
MR-guided focused ultrasound (MRgFUS) thermal therapy has been approved by the FDA for non-invasive transcranial brain ablation to treat essential tremor. However, due to overheating in the skull, the treatment profile of MRgFUS is limited to central locations in the brain. In contrast, histotripsy is cavitation-based and can minimize skull heating by using a low duty cycle (ultrasound on-time/off-time <0.1%). Histotripsy has been shown to treat a wide range of locations within the skull in vitro. Using high-intensity, microsecond-length ultrasound pulses delivered by outside the skull and focused to the target brain tissue, histotripsy generates a cloud of cavitation microbubbles with millimeter accuracy. The rapid expansion and collapse of the cavitation microbubbles mechanically fractionates the target brain tissue into liquefied acellular homogenate. In this work, transcranial histotripsy is performed for the in-vivo pig brain for the first time under MRI guidance. MRgFUS uses MR thermometry to monitor the treatment. As histotripsy is cavitation-based and the tissue damage is mechanical, new monitoring MRI sequences must be developed for real-time guidance. This work describes the real-time MRI method of monitoring histotripsy, with the ablation zone confirmed by post-treatment T2 and T2* images.METHODS
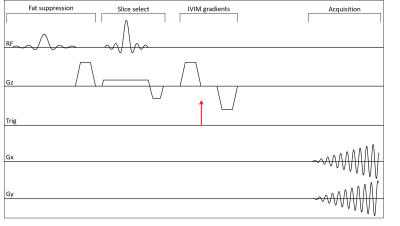
We developed an in-house MR-compatible histotripsy system. The phased-array FUS transducer has 128 elements with fc=700kHz and F-number=0.68. A craniotomy was performed on the pig and cavitation is generated in the brain through an excised human skull at a 10Hz pulse repetition frequency. The transducer was placed in a water bucket with human skull attached through which brain was treated. MRI used a GE MR750 3T scanner with real-time data streaming to a Linux workstation. Figure 1 shows the experiment setup in the MRI scanner and the histotripsy transducer array. Real-time MRI is done using intra-voxel incoherent motion (IVIM) pulse sequence [2,3]. The two diffusion gradients with b = 4 s/mm2 and separated by 4ms to allow dephasing of spins with motion stimulated by the bubble cloud. The ultrasound pulses were triggered by the MRI scanner at the beginning of the 4ms dephasing interval. Pulse sequence diagram is shown in Figure 2. The sequence has TE/TR of 20/500ms with 4 interleaved spiral arms for a volume TR = 2s for 5 slices, FOV=40cm, flip angle 40 degrees and in-plane resolution of 3.125x3.125mm. We also acquired T2 Fast Spin Echo (FSE) (TE/TR = 108/6500 ms, # echoes=19) and T2* (TE/TR = 13.6/600 ms) images of the region of interest before, immediately after and 2 hours after ablation and compared the ablation regions to study the accuracy of real-time monitoring system.RESULTS
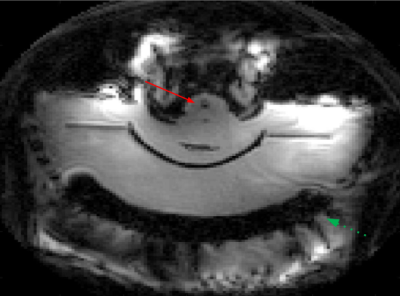
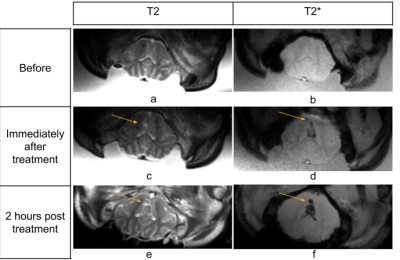
Figure 3 depicts the positioning of the pig brain with respect to the skull. Histotripsy was performed two times on a 3mm3 region in the brain. Figure 4 shows a slice depicting the cavitation in IVIM sequence. The location of cavitation in real-time shows good correlation with the post treatment images and 2 hours post-treatment images as shown in Figure 5. The measured size of the histotripsy lesion was about 4x4 mm in the post-treatment T2 images which is similar to the planned treatment volume.CONCLUSION
In this study, we demonstrated the use of an MRI compatible FUS system and synchronized cavitation using triggers sent from MRI scanner. The real-time images were consistent with post-treatment images, but we note that these were also T2*-weighted, making it difficult to distinguish between blood pooling and IVIM sources of signal loss. Also, due to the large size of the water coupling tank, our FOV was set to 40cm to prevent aliasing and our temporal resolution is about 2 seconds with spatial resolution of 3.125 mm in plane for 5 slices. We plan to develop approaches to address both issues in the future. In summary, this study is the first demonstration of real-time MRI monitoring of histotripsy in vivo.Acknowledgements
This work was supported by NIH Grant 1R01EB028309 and the Focused Ultrasound Foundation.References
1. Zhen Xu, Ludomirsky, A., Eun, L. Y., Hall, T. L., Tran, B. C., Fowlkes, J. B., & Cain, C. A. (2004). Controlled ultrasound tissue erosion. IEEE Transactions on Ultrasonics, Ferroelectrics and Frequency Control, 51(6), 726–736. https://doi.org/10.1109/TUFFC.2004.1304271
2. Bihan, D. Le, Breton, E., Lallemand, D., Aubin, M. L., Vignaud, J., & Laval-Jeantet, M. (1988). Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. https://doi.org/10.1148/RADIOLOGY.168.2.3393671
3. Allen, S. P., Hall, T. L., Cain, C. A., & Hernandez-Garcia, L. (2015). Controlling cavitation-based image contrast in focused ultrasound histotripsy surgery. Magnetic Resonance in Medicine, 73(1), 204–213. https://doi.org/10.1002/mrm.25115
Figures
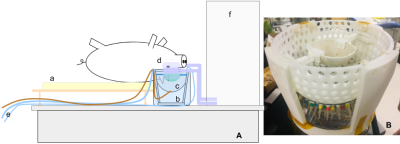
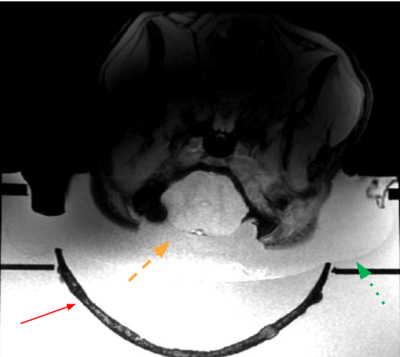
Figure 1. A) Setup of the MR imaging system. The pig lies on its back on a custom-made bed (a) with its head in a water bath (b) which also contains the histotripsy transducer (c). Receive coils are placed on the sides of pig’s head (d). A water pump pumps warm water inside the tank through pipes (e) to prevent water from cooling over time. (f) is the bore of the magnet.
B) Picture of the histotripsy transducer with the skull cap attached on the top.