0104
Rapid autofocusing of MR-guided focused ultrasound acoustic pressure fields using MR-ARFI with spatially coded emissions1Biomedical Engineering, Vanderbilt University, Nashville, TN, United States, 2Vanderbilt University Institute of Imaging Science, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
Magnetic resonance-guided focused ultrasound (MRgFUS) has many potential neurological applications, but skull-induced aberrations of the acoustic pressure field limit its specificity and safety. MR-acoustic radiation force imaging (MR-ARFI)-based methods have been proposed to refocus the pressure field in situ. However, they take too long for practical in vivo use. We propose a multi-voxel MR-ARFI-based autofocusing method for rapid aberration correction of MRgFUS acoustic pressure fields. We compare our proposed method to the canonical single-voxel MR-ARFI-based refocusing method and demonstrate that as few as two MR-ARFI acquisitions can be used to refocus a programmatically aberrated pressure field.
Introduction
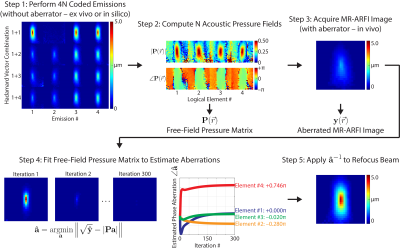
Magnetic resonance-guided focused ultrasound (MRgFUS) has many potential neurological applications.1 However, skull-induced aberrations of the acoustic pressure field limit its specificity and safety.2 The pressure field can be refocused in situ using MR-acoustic radiation force imaging (MR-ARFI), which maps the displacement caused by the acoustic beam’s radiation force.3 Current MR-ARFI-based refocusing methods require 4 spatially coded emissions per element to recover the complex-valued aberrations.4 However, reported scan times (2 hours/512 elements) are too long for practical in vivo use.5 We developed a rapid autofocusing method which can recover the aberrations with many fewer MR-ARFI images by fitting a set of pre-calibrated pressure field maps to the acquired images in a “multi-voxel” magnitude least-squares approach.Methods
An MRI-compatible, 128-element FUS transducer (Image Guided Therapy; Bordeaux, FR) customized for transcranial neuromodulation in non-human primates was used to sonicate a human brain tissue-mimicking phantom (0.5% agar/4% graphite) in a water tank. Sonications were performed at 650 kHz for 10 ms (6500 cycles) at an acoustic power generating a free-field peak negative pressure of 4.8 MPa.Displacement images were acquired using a spin echo 2D MR-ARFI sequence implemented on a 7 Tesla scanner (Philips Achieva; Philips Healthcare, Best, NL), with parameters: FOV/matrix/voxel size 120x120x4 mm3/60x60x1/2x2x4 mm3; TE/TR 29/500 ms; 18° flip angle. A volume head coil was used for transmit/receive (Nova; Nova Medical Inc, Wilmington, USA). Repeated unipolar MEGs were used for ARFI encoding, with gradient duration/strength 10 ms/40 mT•m-1. The second MEG was synchronized with an ultrasound emission using TTL outputs to trigger the transducer. Displacement images were reconstructed by subtraction of four phase images using opposite MEG polarities and with FUS turned on or off: $$$ \Delta d = \angle ((\phi^{ON+} \cdot (\phi^{OFF+})^{*})\cdot(\phi^{ON–} \cdot (\phi^{OFF–})^{*})^{*}) / 2 \gamma G \tau $$$. One displacement image took 2.0 minutes to acquire and required 120 sonications; two dummy displacement images were acquired afterward, resulting in a total duty cycle of 0.4%.
Transducer elements were randomly grouped into 4 logical elements (32 physical elements per logical element), and a known phase aberration pattern ([0, –π/4, 0, 3π/4] rad) was programmatically added to each logical element of the transducer during transmission. The transducer is shown in Figure 1. The “single-voxel” refocusing method (Larrat et al, 2010) was implemented to recover the programmed aberrations using 4 spatially coded emissions per logical element (4 logical elements x 4 MR-ARFI acquisitions per element = 16 MR-ARFI acquisitions or 32.0 min scan time). Though the MR phase was imaged over a 2D plane, this method only uses the mean acoustic intensity across a few image voxels near the acoustic focus (hence “single-voxel” method). Our autofocusing method is summarized in Figure 2. This method can recover the complex-valued aberrations from many fewer MR-ARFI images by fitting a set of pre-calibrated pressure field maps to the acquired images. In the first step, the free-field complex-valued acoustic pressure fields generated by each logical element were computed using the single-voxel method (16 MR-ARFI acquisitions). Next, a set of aberrated MR-ARFI images were acquired. The free-field complex-valued pressure fields were then fit to the acquired images to estimate the aberrations. This is a “multi-voxel” approach that can recover the complex-valued aberrations from the entire MR-ARFI image using magnitude least-squares optimization. The aberrated images used in this fit were those already acquired for the single voxel method. We evaluated the performance of our multi-voxel method using 1, 2, and 8 aberrated MR-ARFI images in the least-squares fit; we computed the aberrations from every candidate combination of spatially coded emissions that were acquired (i.e., $$$\binom{16}{1}=$$$ 16 candidate images for 1 MR-ARFI acquisition, $$$\binom{16}{2}=$$$ 120 candidate images for 2 MR-ARFI acquisitions, and $$$\binom{16}{8}=$$$ 12870 candidate images for 8 MR-ARFI acquisitions) and reported the lowest RMSE that was obtained. In the fit, the free-field pressure matrix was computationally phase shifted by the same phase shift used to generate the spatially coded emission before the aberrations were estimated (so no additional calibrations were required).
Results
Figure 3 compares phase aberration estimates obtained by the canonical single-voxel MR-ARFI-based refocusing method and our proposed multi-voxel method. Our multi-voxel method recovered the programmed aberrations with lower RMSE than the single-voxel method when 2 or 8 MR-ARFI acquisitions were used in the least-squares fit, which represents a 87.5% and 50% reduction in the number of required acquisitions, respectively.Figure 4 shows representative MR-ARFI images acquired using our MRgFUS transducer. When the estimated aberrations were applied to refocus the programmatically aberrated pressure field, our multi-voxel method recovered the acoustic focus with higher peak displacement than the single-voxel method when 2 or 8 MR-ARFI acquisitions were used in the least-squares fit.
Conclusions
A multi-voxel MR-ARFI-based autofocusing method was proposed for rapid aberration correction of MR-guided focused ultrasound acoustic pressure fields. We compared our proposed method to the canonical single-voxel MR-ARFI-based refocusing method and demonstrated that as few as two MR-ARFI acquisitions can be used to refocus a programmatically aberrated pressure field. Future work will evaluate the multi-voxel method using more complex aberration patterns and on our group’s in vivo platform for transcranial FUS neuromodulation in non-human primates shown in Figure 5.6-8Acknowledgements
This work was supported by NIH grants R01 MH 111877, R24 MH 109105, U18 EB 029351, and F31 EB 026928.References
1. Krishna V, Sammartino F, Rezai A. A review of the current therapies, challenges, and future directions of transcranial focused ultrasound technology: advances in diagnosis and treatment. JAMA Neurol 2018;75(2):246-54.
2. Kyriakou A, Neufeld E, Werner B, et al. A review of numerical and experimental compensation techniques for skull-induced phase aberrations in transcranial focused ultrasound. Int J Hyperthermia 2014;30(1):36-46.
3. McDannold N, Maier SE. Magnetic resonance acoustic radiation force imaging. Med Phys 2008;35(8):3748-58.
4. Larrat B, Pernot M, Montaldo G, et al. MR-guided adaptive focusing of ultrasound. IEEE TUFFC 2010;57(8):1734-47.
5. Marsac L, Chauvet D, Larrat B, et al. M. MR‐guided adaptive focusing of therapeutic ultrasound beams in the human head. Med Phys 2012;39(2):1141-9.
6. Yang PF, Phipps MA, Newton AT, et al. Neuromodulation of sensory networks in monkey brain by focused ultrasound with MRI guidance and detection. Sci Rep 2018;8(1):7993.
7. Chaplin V, Phipps MA, Jonathan SV, et al. On the accuracy of optically tracked transducers for image-guided transcranial ultrasound. Int J CARS 2019;14:1317-27.
8. Phipps MA, Jonathan SV, Yang PF, et al. Considerations for ultrasound exposure during transcranial MR acoustic radiation force imaging. Sci Rep 2019 (in press).
Figures
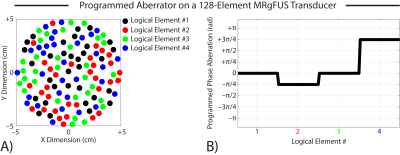
(A) Transducer elements were randomly grouped into 4 logical elements (32 physical elements per logical element).
(B) A known phase aberration pattern ([0, –π/4, 0, 3π/4] rad) was programmatically added to each logical element of the transducer during transmission.
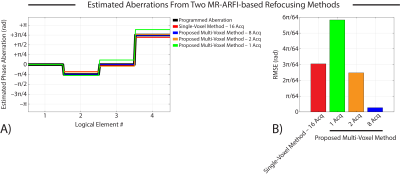
(A) Phase aberration estimates obtained by the canonical single-voxel MR-ARFI-based refocusing method and our proposed multi-voxel method.
(B) Our multi-voxel method recovered the programmed aberrations with lower RMSE than the single-voxel method when 2 or 8 MR-ARFI acquisitions were used in the least-squares fit.
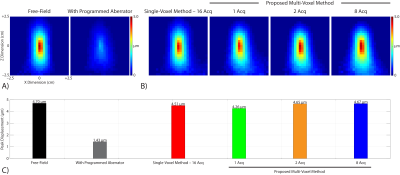
(A) Representative MR-ARFI images acquired using our MRgFUS transducer with a programmatically aberrated acoustic pressure field. The programmed aberrator reduced the measured peak displacement by 70%.
(B) Representative MR-ARFI images acquired using our MRgFUS transducer when the estimated aberrations were applied to refocus the programmatically aberrated acoustic pressure field.
(C) Our multi-voxel method recovered the acoustic focus with higher peak displacement than the single-voxel method when 2 or 8 MR-ARFI acquisitions were used in the least-squares fit.
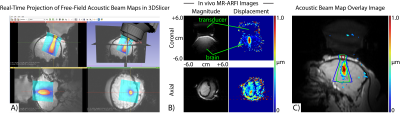
(A) Our group's in vivo platform for transcranial FUS neuromodulation in non-human primates uses 3DSlicer (https://www.slicer.org) to enable real-time projection of free-field hydrophone-derived acoustic beam maps for preoperative stereotactic neuronavigation via optical tracking.
(B) In vivo transcranial MR-ARFI-derived acoustic beam maps confirm that the acoustic beam is targeted in the correct location.
(C) MR-ARFI-derived acoustic beam maps acquired with optical tracking guidance show good agreement with free-field hydrophone-derived beam maps.